We often think of cataracts as a problem that people living in 'developing countries' face and so it may surprise you to learn that every year, around 120,000 people in the UK have surgery to remove cataracts...
Although cataracts result from many conditions, the most frequent cause is the natural aging process. Other causes can include injury, chronic eye disease and diseases such as diabetes. More than half the people over age 65 have some degree of cataract development and cataracts can take from a few months to several years to develop.
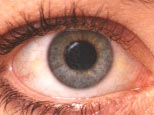 | 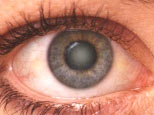 | |
a. Eye without cataract | b. Eye with cataract | |
Figure 1 * | ||
A cataract is a clouding of a part of the eye known as the lens Cataracts form when the transparent lens in the eye becomes cloudy. The lens is the part of the eye responsible for focusing light and producing clear, sharp images and is contained in a sealed capsule. It is a remarkable structure, containing a very high concentration of some of the longest-lived proteins in the body. In fact, the proteins that you are born with in the lens are present throughout your life. Over time or with external damage, these proteins may start to clump together, causing the lens to cloud and making images look blurred or fuzzy. This results in visual impairment or blindness and can even occur during infancy and early childhood. Currently there are no proven drugs to prevent or cure cataract, but the problem can be cured by surgery. The development of novel treatments for cataract are therefore much needed, but this is currently hindered by our incomplete understanding of the processes of cataract formation in lens cells and the underlying cause of this disease at the molecular level.
The eye lens The lens is an organ that, remarkably, retains all its cells for the lifetime of the organism. It is enclosed in its collagen capsule and is bathed in fluids and so is completely avascular (lacking blood vessels). The lens fibre cells that make up the bulk of the lens structure are post-mitotic and the differentiation process involves the complete removal of all cellular organelles including nuclei, making these fibre cells incapable of protein synthesis, DNA replication and RNA transcription. Although there are many other post-mitotic cells in the body that are equally long-lived, none of them survive for decades without these key organelles. Having lost most of their internal machinery, lens fibre cells can be thought of as a protein-filled "sack" in which a loose liquid-like arrangement of proteins enable transparency (see figure 2).
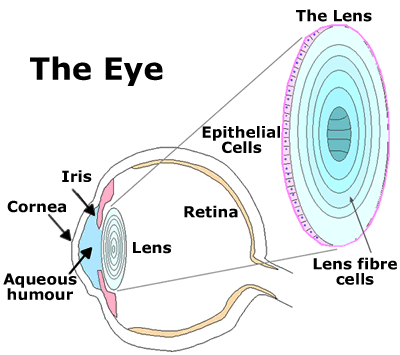 |
Figure 2 - The eye lens |
The eye lens not only contains some of the oldest cells in an organism, but also the oldest proteins since, in the centre of the lens nucleus, there is no protein turnover during the lifetime of an individual. Thus, there is a requirement for both stability and longevity of lens proteins, the majority of which belong to a single family of proteins called the crystallins. (These proteins are called "crystallins" because the ancient Greeks considered the lens to be "ice-like" in appearance). This extreme biological situation has led to the selection of very stable proteins and also to an enrichment of protein chaperones in the lens. The term "chaperone" has been borrowed from Victorian escorts and, as the term implies, refers to the ability of these proteins to associate with other proteins. Stress conditions such as heat, oxidation and exposure to heavy metals result in destabilisation of proteins, which can lead to aggregation and precipitation; chaperones recognise and bind to these non-native protein conformations and prevent aberrant protein-protein interactions.
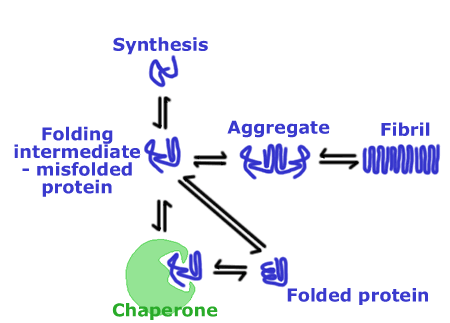 |
Figure 3 - Protein chaperones |
The major protein chaperones that are expressed at high levels in the lens are the a-crystallins and these may serve a protective role in the lens by binding to unfolded proteins, preventing precipitation through the formation of a stable complex. As a result, any potential contribution of a precipitate to cataract formation may be minimised. The lens therefore appears well equipped to deal with any aberrant forms of proteins that might develop and perturb its properties. Nonetheless, lens function can be abrogated by the formation of either inherited or sporadic lens cataracts (see figure 3).
Mutation of crystallin protein is linked to cataract
The g-crystallins were characterised as major lens proteins over a century ago and several mutations in the g-crystallin genes that lead to cataract have been identified in mice and humans. In preliminary work Cait's group (in a major research effort involving groups both in the UK and overseas) have demonstrated that cataract formation in mice that have a mutant form of g-crystallin is due to the formation in the eye lens of structures known as amyloid fibrils. Amyloid fibrils are very stable protein aggregates well-known to medicine: they are involved in a wide range of diseases including Alzheimer's disease and type-II diabetes. In the case of the mutant mice, the normally liquid-like "sack" of the lens fibre cell becomes enriched in fibrous aggregates (see Figure 4). Although the role of amyloid fibrils in diseases like Alzheimer's disease is unclear, in the case of cataract the most likely role of the aggregates is simply to scatter light, causing lens fogging.
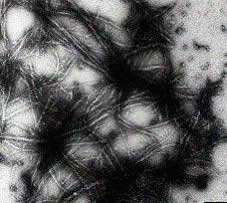 |
Figure 4 - The formation of amyloid fibrils by a mutant g-crystallin |
Future research goals
Cataracts do not, however, just occur in people (or mice) with a mutation in one of their crystallin proteins. Indeed, the vast majority of cataracts are age-related, and do not appear to have anything to do with inherited mutations. Cait's group is now working to discover whether "normal" eye lens proteins - the proteins in the lenses of most of us - also form these amyloid fibrils. This is a viable hypothesis since many of the environmental factors linked to cataract - diabetes, UV exposure, contact with chemicals - are known to also change the structure of proteins and may therefore drive the formation of fibrous aggregates. If this is indeed the case, then age-related cataract can be placed in the amyloid family of diseases, and many of the therapeutic strategies under investigation to prevent amyloid fibril formation in diseases like Alzheimer's disease may also be of use to cataract sufferers.
ABOUT THE AUTHORS:
Dr. Cait MacPhee is a biophysicist and a Royal Society University Research Fellow at the University of Cambridge Cavendish Laboratory.
Dr. Karen Smith is a neuroanatomist and the Business Development Manager for Life Sciences at the University of Cambridge Corporate Liaison Office (CLO).
ACKNOWLEDGEMENTS:
* Courtesy of St Lukes Cataract and Lazer Institute
|











Comments
Add a comment