In 2011 it was estimated that more than one in three people in the UK will contract some form of cancer during their lifetime [1]. Until a cure is found for all types of cancer (in an ideal world) the single greatest advancement would be early detection- catching a cancer when it is in its most treatable stage. But could glass help us do this?
Brief history of glass...
 Glass has become so common that for the majority of us it is easy to overlook its existence; however this has not always been the case. Although some isolated findings date back to 7000BC, it is clear that by 2500BC people have become fascinated with its transparency and brilliance [2]. A book published by Antonio Neri in 1612 titled 'L'Arte vetraria (The art of glass)' contains the description: "Glass...is much more gentile, graceful, and noble than any metal,...it is more delightful, polite, and slightly than any other material at this day known to the world". Certainly this was the view taken by early glassmakers whose only concerns were making decorative objects that mimicked gems and semiprecious stones. Such objects were often found in the tombs of Pharaohs alongside their golden death masks. Although we are not entirely sure how and when humans first discovered the procedure of making glass, one theory suggests that the combination of sea salt (NaCl) and bone (CaO) found in the embers of a fire built on a beach (a source of silica) could sufficiently decrease the melting temperature of sand and hence produce a crude form of glass. With such an early advent of production it is surprising that the concept of glass blowing was not introduced until the first century BC which transformed the formally ornamental material into one with purpose, providing a larger range of applications. Furthermore, the start of 'glass science' was only introduced in 1886 by Otto Schott and Ernst Abbe with their publication on soda-lime-silica glasses and compositional changes.
Glass has become so common that for the majority of us it is easy to overlook its existence; however this has not always been the case. Although some isolated findings date back to 7000BC, it is clear that by 2500BC people have become fascinated with its transparency and brilliance [2]. A book published by Antonio Neri in 1612 titled 'L'Arte vetraria (The art of glass)' contains the description: "Glass...is much more gentile, graceful, and noble than any metal,...it is more delightful, polite, and slightly than any other material at this day known to the world". Certainly this was the view taken by early glassmakers whose only concerns were making decorative objects that mimicked gems and semiprecious stones. Such objects were often found in the tombs of Pharaohs alongside their golden death masks. Although we are not entirely sure how and when humans first discovered the procedure of making glass, one theory suggests that the combination of sea salt (NaCl) and bone (CaO) found in the embers of a fire built on a beach (a source of silica) could sufficiently decrease the melting temperature of sand and hence produce a crude form of glass. With such an early advent of production it is surprising that the concept of glass blowing was not introduced until the first century BC which transformed the formally ornamental material into one with purpose, providing a larger range of applications. Furthermore, the start of 'glass science' was only introduced in 1886 by Otto Schott and Ernst Abbe with their publication on soda-lime-silica glasses and compositional changes.
But what is glass?
The most recent and reliable definition describes glass "as an amorphous solid completely lacking in long range order, periodic atomic structure, and exhibiting a region of glass transformation behaviour". This explains that any material of organic, inorganic or metallic origin can be made into a glass via any technique as long as it presents what is called a 'glass-transition temperature range'. Therefore, contrary to many beliefs, silica (SiO2) is not a mandatory component of glass. This article will in fact focus on a relatively new type of glass called the 'chalcogenides' which are distinctly different in appearance from our more common silica glasses, and have the remarkable ability to transmit light in the mid-infrared (MIR) region of the electromagnetic spectrum- a property most attractive for cancer diagnostics.
Chalcogenide glass
Chalcogenide glasses are so named because they contain one or more of the chalcogen elements found in Group 16 of the Periodic Table: sulfur (S), selenium (Se) and tellurium (Te). Group 16 also contains oxygen which forms another group of glasses known as 'oxide glass'. As you would expect, oxide compounds contain at least one oxygen atom and one other element; a well-known example being carbon dioxide (CO2) formed from carbon and oxygen. Similarly, 'oxide glasses' will contain at least one oxygen atom with the most common type being silicon dioxide (SiO2), frequently known as silica. Although chalcogenide glass and oxide glass belong to the same material group their appearances are remarkably different and as the following pictures demonstrate, the grey-opaque nature of chalcogenides is far removed from the kind of silica glassware you would expect to find on your dinner table. This opaqueness is key to its use in cancer detection.
  |
|---|
Why do chalcogenide glasses appear grey and opaque?
The arrangement of atoms within a material has a profound effect on whether the object will be transparent in visible light; in particular it is the organisation of the electrons around the atoms that dictates this. Electrons can either be in a ground state (normal state) or in an excited state, and never in between. The energy required to move to an excited state can come in the form of a photon (a particle of light) shown in the diagram below.
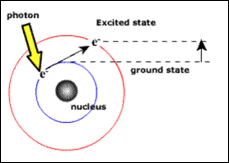 |
|---|
When a visible light photon (i.e. a photon which we can see) travels through silica it doesn't have enough energy to move the electron to its higher energy level, instead the photons will continue travelling through the glass and the material will appear transparent. However, when a visible light photon travels through chalcogenide glass, there is sufficient energy to excite the electrons and so it is absorbed. This stops the photon travelling any further and the chalcogenide glass appears opaque in visible light.
So far we have considered what happens when visible light travels through the two glasses however, there are other types of light which humans can't see, some with longer wavelengths and some with shorter. The different types are illustrated below in the electromagnetic spectrum.
Electromagnetic spectrum
 |
|---|
Imagine if humans could see infrared light found just beyond the red side of the rainbow, in particular mid-infrared (MIR) light in the spectral region of 3-25 μm. The chalcogenide glasses would now appear transparent because the MIR photons no longer have enough energy to excite the electrons and so they will continue travelling through the glass; i.e. chalcogenide glass can transmit MIR light. In contrast, the silica glass would now appear opaque because there is sufficient MIR photon energy to excite the electrons, preventing the light from travelling any further.
How does this help us find cancer?
Most types of cancer have four stages (five if you consider 0 to be a healthy patient). Stage 1 means the cancer is small and is contained within the organ of origin; stage 2 sees an increase in size but is still contained within one organ; stage 3 means the cancer is now large and is likely to have spread to surrounding tissue (potentially cancerous cells in the lymph nodes); and finally stage 4, the most critical, where the cancer has travelled to other areas of the body.
At present, the most common form of diagnosis uses visible light microscopy to analyse a biopsy (an extracted tissue specimen) stained with a dye. However, this procedure is lengthy and often requires the patient to travel to a regional hospital. In 2012 more than a quarter of all deaths in the UK were caused by cancer[3] and as recent figures suggests almost half of those patients will be diagnosed in either stages 3 or 4, resulting in an estimated survival of 5 years[4]. Evidently there is increasing demand for a technique that can quickly detect cancer in its early stages when the disease is more treatable; this is where chalcogenide glass fibres become effective.
The MIR spectral region contains a very important section called the 'molecular fingerprint' and is unique to every single molecule. Detecting this characteristic region could provide an instrumental tool for early diagnosis because the state of a cell -normal or diseased- is subtly shown through changes in the MIR spectrum. The diagram below illustrates this point with an MIR light source at 1, which travels through the molecules at 2, before a specific spectrum is detected at 3. Chalcogenide glasses will be fundamental to this operation as they are able to deliver and collect MIR light to and from the tissue.
 |
|---|
Efforts have already been made to map these changes using a method called Fourier transform (FT) MIR spectroscopy. So far it has been able to image biological tissues in a highly sensitive manner which is non-destructive and has no need for dyes. However, the light source which is used for this is weak and still necessitates the removal of tissue from the patient. New research however, has proven the ability to produce chalcogenide fibres which give a much brighter MIR source [5]. This technology will allow in vivo (in the body) analysis of the tissue thereby providing real time information regarding the cellular components, metabolic processes, organ and cell architecture and therefore in turn revealing the absence/presence of a cancer. This novel procedure would provide immediate diagnosis of the tissue, drastically reducing the waiting time for the patient, and hence improving their chances of survival.
References
- Previous Watching the skies
- Next The parasites that control your mind









Comments
Add a comment