Uncovering schizophrenia through skin
Interview with
Hannah - In this episode, I'm reporting from the British Association of 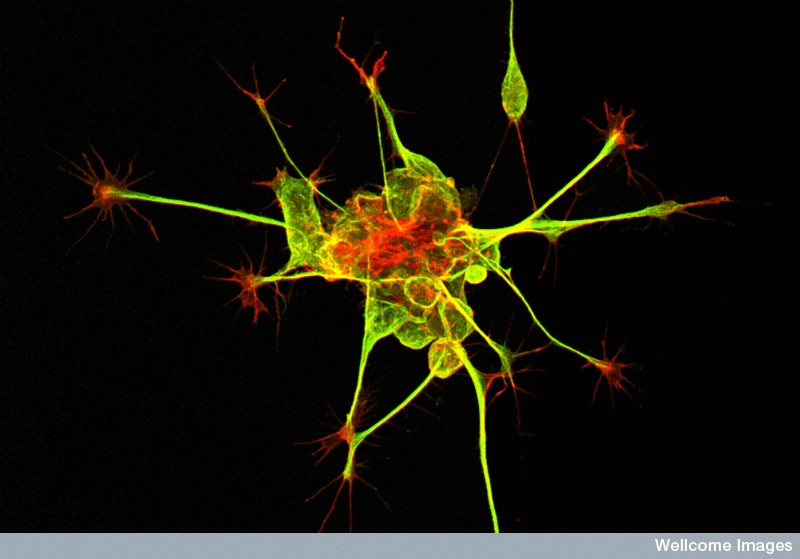 Psychopharmacology 2014 Meeting in Cambridge. Does smoking dope decrease your potential for pleasure? Now, the stereotypical stoner is often portrayed as an unmotivated apathetic armchair philosopher. A new research reveals the biological underpinnings for this with chronic marijuana use, rewiring the brain, making it less sensitive to the motivation and reward chemical, dopamine.
Psychopharmacology 2014 Meeting in Cambridge. Does smoking dope decrease your potential for pleasure? Now, the stereotypical stoner is often portrayed as an unmotivated apathetic armchair philosopher. A new research reveals the biological underpinnings for this with chronic marijuana use, rewiring the brain, making it less sensitive to the motivation and reward chemical, dopamine.
Michael - And when people smoke lots and lots of Cannabis over time, the dopamine system can become used to being stimulated. And so, it tries to adapt.
Hannah - How the simple act of dieting can rewire the happy brain chemical serotonin, making you more compulsive and increasing your susceptibility to developing an eating disorder.
Philip - And without meaning to, if you lower brain serotonin levels through dieting, that can lead to lower mood and more compulsive behaviour. So, the dieting really gets a grip on you and it's very hard to stop.
Hannah - And depression in teenagers...
Lesley - Estimates vary, but probably around 5% to 6% of adolescents have moderate to severe depression.
Hannah - The shocking stats and what we can do to help lift their mood.
First up, the Naked Scientists Kate Lamble met Dr. Kristen Brennand from the New York Stem Cell Foundation. She's been punching into people's skin, and using clever chemistry. She converts the skin cells that she picks up into brain cells which she grows in a dish, all part of a quest to unravel the mysteries that surround the condition, schizophrenia...
Kristen - So, Schizophrenia is a really common and really debilitating disorder. It affects about 1% of people across the world in every culture and every country. And those who are affected, the common symptoms are hallucinations and delusions. There are of course many, many other symptoms but that is the classic phenotype.
Kate - What goes on in the brains of people with Schizophrenia? Do we know what causes these symptoms?
Kristen - So, that is actually a really complicated question. Actually, just this morning, a paper came out in a really famous journal Nature, showing that there are now 108 genes that have been implicated into Schizophrenia. So, we know at least 108 ways that somebody can be Schizophrenic and the answer is probably even more than that. It seems like the brains of patients on the whole are affected. In post mortem studies, we begin to see that the brains are smaller and in functional (fMRI) imaging studies of live patients, we can see their brains don't connect as well. But the short answer is no. we don't know all the ways that things are going wrong in the brain are people with Schizophrenia.
Kate - Why is it so important that we unpick this problem? Is it really important for treatment?
Kristen - Well, I think it's really important for treatment. First aside as a whole, as I said, any time 1% of the people. There's 100 million people around the world are affected by a disease. You really want to understand why and treat it. this is a really debilitating disease. 95% of patients who are diagnosed with Schizophrenia will never again will have a full time job. 50% will have drug and alcohol abuse problems and in the US at least, 1 in 5 will be homeless and live in the streets. So, this is a really important disease that has no cure that it would be wonderful if we could get insights in new medications to help these people.
Kate - So, you've been using stem cells to help unpick this problem. How have you gone about looking at that?
Kristen - In 2006, a remarkable discovery was made by a researcher in Japan by the name of Shinya Yamanaka. What he showed is that you could take skin samples from any person on the planet and reprogramme those into stem cells. Stem cells of course can become any cell type in the body or at least this type of stem cell can be. And so, what I've done is I've taken skin samples from healthy controls and from patients with Schizophrenia and turn them both into stem cells. And then those become brain cells - neurons - that I can compare. And so, we started by asking really basic questions - how are the brain cells different between patients with Schizophrenia and healthy controls? And we've begun to see a few differences. The second question is, why are they different? That's really the hard question. It's, why are these cells different and how can we make them the same.
Kate - When you take stem cell and you turn it into a neuron, is that a good model for neurons that are found in adult brains or are they slightly different?
Kristen - They're more than slightly different unfortunately. We have been doing gene expression comparisons of neurons from our stem cells and what I can tell you is when we compare them to neurons in human tissue, they're most similar to first trimester foetal tissue. So, our cells are really young. Nonetheless, they fire action potentials, they form synapses so the cells that we make from stem cells are functionally similar to the neurons in the human brain. But there are some really important limitations. They're not myelinated like those in the brain might be and they're heterogeneous. So, they're not all of one cell type. As a consequence, they wire in ways that they don't typically wire in the brain. So, it's a model. It's an imperfect model, but it's at least a source of live human neurons to study.
Kate - So, when you look at the differences between the neurons that you've created from controls and the neurons that you've created from those with Schizophrenia, what are the differences that you start to see?
Kristen - There are a few major types of differences that I and other groups have seen. Some of the commonalities that we see is the neurons don't connect to each other as well. So, they have fewer branches, fewer dendrites growing from the soma. They have fewer synapses along the dendrites and functionally, some groups unfortunately - not mine, but other really great groups have shown that the synapses are not as functional. They don't fire as often between the neurons. Collectively as a whole, I think the field has shown poor connectivity, reduced synaptic activity and structure, increased oxidative stress and aberrant migration of Schizophrenia in neurocells.
Kate - So, the cells from people with Schizophrenia were acting slightly differently, but there are these 108 gene that might play a role in that. Do we have any idea what's causing that?
Kristen - It's likely that in every patient, their cellular phenotypes might be coming from different mutations. In fact, I've genotyped my patients and they don't share the same mutations. So, it seems like collectively, pathways are being effective. They have the same downstream consequences but the genetic root is actually different in each patient.
Kate - So, does noticing these changes, does that help us to develop drugs which might solve them?
Kristen - So, that's actually the ultimate goal - is to have a drug screening platform. I can make a limitless number of neurons from any patient with any genetic background. And so, the hope would be that the geneticist can collectively sort patients into maybe 500 or a thousand different ways of having Schizophrenia and that we could then do a drug screen for each of the thousand different ways that one could be a Schizophrenia. And so that your doctor will ultimately might say, "You're type 208. You should use this drug and this dose." And so, that's I think the ultimate place that the stem cell originally wants to get to is drug screening.
Kate - So, this is sort of going into the personalised medicine idea. You'll know exactly what type you have of the hundreds and then they wish drugs will be able to help you.
Kristen - It's a very similar approach to what people are doing for cancer right now. Yes, the goal is to better predict what drug will help and to avoid the side effects from those that don't.
Hannah - Thanks to Kristen Brennand speaking with Kate Lamble.









Comments
Add a comment