Off the wall
Interview with
Many bacteria are clad in a tough cell wall that provides protection and also 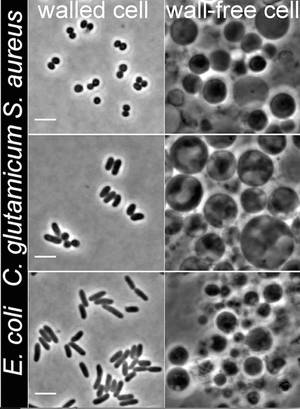 stops the cell from bursting through osmosis. The cell wall's also critical for bacteria to divide correctly, or at least that's what we thought. Because now, Newcastle scientist, Jeff Errington has found that by growing bugs in antibiotics for an extended period and then deleting a small clutch of genes, it's possible to produce strains called L-forms that lack a cell wall but can, critically, still reproduce, albeit very slowly. This could be the way their ancestors did it early in evolution. But here in the present, it could now hold the key to producing self-replicating synthetic cells.
stops the cell from bursting through osmosis. The cell wall's also critical for bacteria to divide correctly, or at least that's what we thought. Because now, Newcastle scientist, Jeff Errington has found that by growing bugs in antibiotics for an extended period and then deleting a small clutch of genes, it's possible to produce strains called L-forms that lack a cell wall but can, critically, still reproduce, albeit very slowly. This could be the way their ancestors did it early in evolution. But here in the present, it could now hold the key to producing self-replicating synthetic cells.
Jeff - The cell wall is incredibly important for bacteria and one of the things that it does is to protect the cell from bursting due to the osmotic pressure inside the cell which pushes out on the cell membrane. So, normally bacterial cells rely on the wall for survival. And one of the reasons why antibiotics work so well is that they interfere with the synthesis of the cell wall, and in conditions where a cell wall isn't formed, the cells basically explode. So, we have to establish conditions where we could protect the cells from this sort of explosive event. And what surprised us a little bit to begin with was that the cells that had no cell wall would simply sit there without growing.
Chris - Were these cells actually viable? Could you tell whether they were alive or were they just sitting there not doing anything because they were dead?
Jeff - They're actually alive. You can infect them with viruses, for example, and they'll support the growth of these viruses. They simply don't grow and actually, we don't yet understand why they don't grow. But what we discovered was that if we select for certain mutational events then the cells would grow.
Chris - What are the mutations that are necessary to make these bugs propagate then, and how do they make a difference?
Jeff - Well. The key mutation that we discovered led to a single kind of event that then forced the cells to produce excess amounts of cell membrane. So, they result in increasing the surface area of the cell relative to its volume and surprisingly to us, it turned out that that is sufficient for these cell wall division bacteria to grow.
Chris - Do you have any idea why just having an excess of cell membrane would have that dramatic effect?
Jeff - This project has taken us to very interesting places around various aspects of biology but also to biophysics. And it turns out that there are biophysicists who are working on trying to mimic how they imagine very primitive cells back in the early days of life on the planet would have grown and divided. And the biophysicists had already been thinking that by increasing the surface area of a simple membrane bag, that that might drive the proliferation of those cells.
Chris - That's very surprising, isn't it? That these bacteria should still be able to divide in this way because is there not a very highly evolved mechanism which involves various skeletal elements inside the cells that enable the bacteria to make sure that each of the daughters have got copies of the chromosomes and so on. So, they must be ditching that mechanism in order to still be able to divide in this L-form that you've created.
Jeff - That's right. Probably the biggest surprise that we got very early on - the whole of the cell division machinery that bacterial cells normally use, becomes completely dispensable in the L-form cells. So, we can delete many of the genes that are normally required for division and it makes absolutely no difference to the proliferation of the L-forms.
Chris - Putting this all together then, you have identified the genes which appear to be necessary for you to deactivate. This in turn, leads to a loss of the cell wall, an overproduction of cell membrane, and this restores the ability to these cells to be able to divide by a mechanism as yet we don't really understand. Why is this important, though? And can we do this with any bacterial cell if instead of bacteria subtilis, Staph. aureus, for example. Could we do the same thing with them?
Jeff - The basic principles of L-form biology that we've uncovered seem to apply also to a very wide range of bacteria. In the new paper, we show that Staphylococcus aureus and E. coli seem to be able to undergo very similar L-form switch. And all of this is important for several reasons. There are many clinical case studies implicating L-forms in all kinds of diseases, many of a chronic or persistent nature. One of the reasons why L-forms might be implicated in those diseases is that antibiotics like penicillins are absolutely ineffective against L-forms.
Chris - What about another potential implication and, perhaps, application which is that have heard from the same crew, Craig Ventner, who sequenced the genome in recent years are now saying they made semi-synthetic life. Could we also use this sort of approach in making synthetic microorganisms or just synthetic cells in general?
Jeff - We're very excited by the possibility of using what we've learned about L-forms in that sort of context. It seems to me to be quite daunting to imagine how you could build a machine that would synthesize the wall in the correct configuration and then a division machine that would bring about the accurate division of walled cells. But of course, we now know with the L-forms that we can have pretty normal, in a sense, cells growing in the complete absence of the whole cell wall synthetic machinery and the whole of the cell division machinery. So, that makes the prospects of generating an artificial cell, I think, rather more straight-forward.
- Previous Horizontal gene transfer
- Next The one and only









Comments
Add a comment