The Search for New Drugs
Interview with
Chris - Joining us in the studio this week is Dr Harren Jhoti who is the founder of the drug discovery Astex Therapeutics. He set this up in 1999. He started off as the chief scientific officer and he's now the chief executive officer. Harren, thank you for joining us. What's the basic way in which, before you came along with your techniques, we were trying to invent new drugs?
Harren - The conventional approach to discover new drugs really starts with initially finding a target which may be associated with a particular disease. That target, quite often, is a protein which malfunctions in one way or another. Sometimes it's hyperactivated the protein's function. What you then do is you take that protein and then you screen it against a collection of compounds. Basically these are libraries of chemical entities. Quite often these libraries are really rather huge in the sense there could be hundreds of thousands of different compounds. The aim there is to identify some compounds which actually bind or interact with that target protein. Those are called leads in the drug discovery terminology. After that what happens is you try to optimise those leads by doing some iterative chemistry, i.e. you try to improve the interaction of those compounds against the target protein. You try to impart in those compounds the drug-like properties one needs for that compound then to become a drug.
Chris - In the real world how long would it take between you coming up with a structure for something in the body you want to target. Say you find a gene which makes something linked to, say, high blood pressure and you want to make a drug to lower blood pressure by blocking that gene. How long would it take if you invented a molecule between the invention of that molecule, the conception in the laboratory, and it actually going into a patient? What's the average time?
Harren - You know, this process and procedure is a really very long time. It takes many years and it ranges between five to ten years often to go from the concept to a drug which may get onto the market place and to treat patients. That's why it requires a huge amount of investment in this whole process.
Chris - What's the average price tag for a drug coming out today?
Harren - The kind of monies it requires to develop a drug is in the order of $800 million.
Chris - Wow.
Harren - That number also does include a lot of the programmes which actually fail to generate the compound.
Chris - So that's why pharmaceutical companies when they make a drug have to charge a lot for it? Certainly to the first world, in order to make their money back to bankroll the development of other drugs that are not going to succeed?
Harren - That's exactly right. The actual failure rate is really quite eye-watering, in fact. Depending on where you look at the process, what the metrics are. They range in the order of one in 100 or one in 1000 compounds actually get to the market place.
Chris - Why do most not make it?
Harren - That's a big question and a lot of people are trying to understand how we can improve this productivity. One thing which is quite clear from looking at the types of chemistries which have been generated using this more conventional approach is that perhaps these molecules have become too molecularly complex. Perhaps they're a little bit too large. What happens then in the body is that some of these compounds are actually metabolised in a negative way which generates toxicities.
Chris - So you're saying the actual drug that the company might make ends up being far too big and ends up turning into other things in the body which can have harmful effects. That's why the compound fails?
Harren - There is clear evidence which suggests that the attrition or the failure rate of compounds which are being developed in the clinical setting is correlated with the size of the molecule.
Chris - Do you think it's also correlated with the size of the legal bills that companies like Merck have been facing? When they come up with a drug and then get sued for a billion dollars a few years later because of consequences which came to light later this is probably a massive disincentive, isn't it?
Harren - There is no question these are broader societal pressures on the industry. I think in a very general way society has perhaps become a little bit overly risk-averse. There's no question every drug will be toxic to some degree, depending on the dose. The whole question here, or the challenge, is to give appropriate amounts of drug to deal with the disease and not generate toxicity.
Chris - What's the approach you've been taking at Astex to do it differently and surmount some of these difficulties?
Harren - The key issue that we've been trying to target is to try to keep the eventual drug candidate, the drug molecule, slightly smaller - slightly less complex. What we do is, rather than screen these compounds which are quite often between 300-500 Daltons in size we actually screen much smaller chemistry: between 1-200 Daltons. [1 Dalton is approximately the mass of a hydrogen atom] These are what we call fragments. Then what we're able to do is add chemically to those fragments to improve the interaction of those fragments to the target protein.
 Chris - Still sounds like an enormous amount of work, though. How are you able to do this and do it more efficiently and more cost-effectively than other companies? If it was that easy they'd be doing it.
Chris - Still sounds like an enormous amount of work, though. How are you able to do this and do it more efficiently and more cost-effectively than other companies? If it was that easy they'd be doing it.
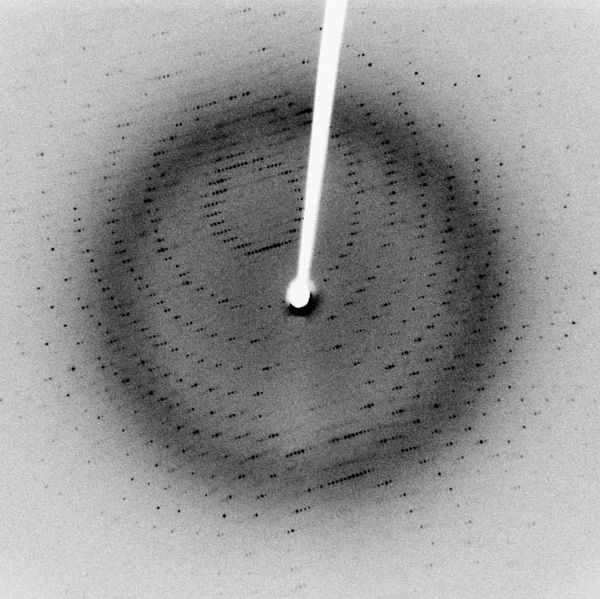
Harren - There's no question it is difficult. What we've been doing is focussing on how you would optimise the technology to be able to firstly detect these very small fragments which is a technical challenge in itself and then to optimise. What it really boils down to is visualisation of how these fragments bind. We've developed biophysical techniques: x-ray crystallography, NMR and integrated the suite into an approach which we call Pyramid to allow us to do this fragment-based drug discovery.
Chris - Talk us through the Pyramid bit-by-bit, exactly how you would go about your technique for making a new agent.
Harren - Once we've identified the target protein we then produce the three-dimensional structure in that protein by doing x-ray crystallography. For that you have to do a lot of protein expression and biochemistry.
Chris - So that gives you the shape and what the molecule actually looks like. How does that help you?
Harren - That helps in two ways. Firstly, it tells us exactly where a fragment has been bound to that target and that allows us to rationally optimise that fragment to fit better into that target protein.
Chris - So that gives you the business-end of the drug so you know which bit is doing the important job?
Harren - That's right. Then what we could do really is what we're doing is hand-crafting these molecules to very uniquely directly fit into the pockets of the protein. The other key advantage of his approach is that these fragments are very low affinity. They bind with very low interactions and it turns out that biophysical techniques such as x-ray crystallography and NMR are really much better ways of detecting the original binding of the fragments.
Chris - Then where do you go?
Harren - What we then do is do several cycles of optimisation. We add extra functional groups onto these fragments and grow these fragments out, very much like a seed. Like planting a seed and growing a seed. Once we actually have molecules which have the appropriate profile in terms of the properties one requires from a drug transit these molecules are then tested in human beings. Of course before you do that you have to test them in preclinical models.
Chris - Once you're arrived at this molecule you want to make how do you actually get it so you can make it efficiently? If you end up with very complicated chemistry and needing to make these very complicated but small molecules, how do you make them?
Harren - It turns out actually the fragments are very simple, much more simple, than the larger complex molecules which conventional screening or conventional drug discovery actually use. The chemistry's simpler and less chemically challenging than conventional drug study.
Chris - Have you got drugs that are actually out there in the clinic or which are just experimental at this stage?
Harren - We've put three compounds now into clinical trials. Our particular focus today is developing the experimental cancer therapies. We have three compounds being tested in cancer patients: both in the US and in the UK.
- Previous Keeping Tabs on Jellyfish
- Next Discovering Drugs from Bugs









Comments
Add a comment