Ingredients
 | 2 similar sized large balloons |  | A short piece of tube, slightly larger than the necks of the balloons. Some marker pen lids work nicely, or a piece of hose |
Instructions
Stretch the neck of a balloon over one end of the tube.
Blow up the balloon through the tube so it just starts to stretch.
Twist its neck to keep the air in
Stretch the other balloon's neck over your tube.
Untwist the neck and see what happens.
Can you get the two balloons to stay at the same size?
Result
The big balloon won't inflate the small one, and it should be very difficult to get both balloons to be the same size.
Explanation
If you have ever tried blowing up a balloon you will probably have noticed that to start blowing it up you need a huge pressure but once it is started it becomes quite easy until it is fully inflated when it gets hard again. So when balloons are just starting to inflate easily, a smaller balloon will be harder to blow up (at a higher pressure) than a slightly larger one. So if they are connected air will flow from the small to the large balloon increasing the difference in size.
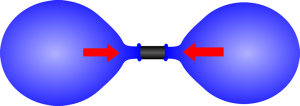 | 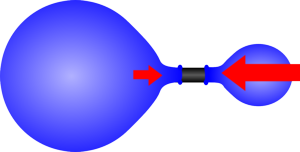 |
| If the two balloons are almost the same size the larger one will be at a lower pressure. | As one gets smaller the pressure increases so it shrinks until it reaches its unstretched size. |
Why is the pressure lower in the larger balloon?
There are two effects which cause this. The first is connected to the shape of the balloon. The small balloon is more curved so more of the tension in the rubber is compressing the gas, so for the same tension there will be a higher pressure in a smaller balloon. This is why blowing up long thin balloons is so difficult, the bit at the end is very curved so the pressure is very high.
If you think of a little bit of rubber and the direction that the tension is pulling in, on a curved piece of rubber the tension is pulling the rubber downwards compressing the gas, but on a less curved piece the rubber is mostly pulling against other rubber and not squashing the gas inside.
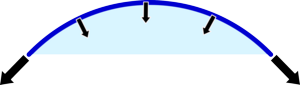 |  |
| If the balloon is small then it is very curved so quite a lot of the force is compressing the gas in the balloon. | If it is larger then much less of the tension in the rubber is directed to squashing the gas. |
The other effect is a peculiarity of rubber. if you stretch a rubber band, it starts off stiff, then gets more stretchy and then goes very stiff again, If you half inflate a balloon it is just starting to get more stretchy. So if one balloon is slightly smaller than the other the rubber will be stiffer on the smaller balloon making the pressure even higher.
If you try and do the same experiment with fully inflated balloons it won't work because they will both get stiffer as you stretch them so the larger balloon will be at a higher pressure and air will flow to cancel out any differences.
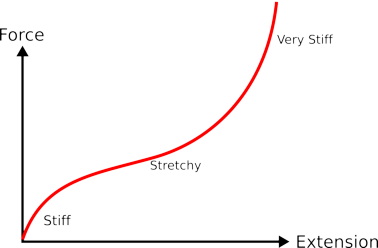 |
| if you stretch a piece of rubber it starts off quite stiff then as it gets thinner it gets less stiff, but as you stretch it even more it gets very stiff. |
These two effects combine to explain why it is so hard to start blowing up a balloon, then easy then hard again.
Why does rubber behave so strangely?
Rubber is made up of lots of long string like molecules occasionally attached together ( crosslinked ) occasionally to form a very widely spaced random and tangled net. As you start to stretch it the rubber band gets thinner so it gets less stiff, but as you continue to stretch it the rubber molecules start to become straight so they can't stretch any more, and the rubber gets stiffer again.
What has this got to do with bubble bath?
You may have noticed that foam doesn't last forever. One reason is that the bubbles eventually burst, but there is another effect which encourages this and is connected to this experiment. Bubbles are blobs of gas surrounded by thin layers of water. This water has surface tension which is trying to make it as small as possible, a bit like the rubber in the balloon, but water has an even more strange force extension curve. The force is a constant whatever the extension, So the only thing which affects the pressure inside a bubble is how big it is, a small bubble will be at a higher pressure than a larger one. This means that gas will tend to leak through the walls (by diffusion) from the small bubbles to the larger ones, making the small ones smaller and the large bubbles bigger. So over time you get fewer and fewer larger and larger bubbles.
;
| |  |
| A foam trapped under a plastic sheet and speeded up 25x. Ignore the popping bubbles, just watch a small bubble, it should be shrinking. | The small bubbles are at a higher pressure so air will leak out of them |
- Previous Vacuum Powered Bazooka
- Next Harmonic Knives









Comments
Add a comment