Why is your breath sometimes warm and sometimes cold
Ingredients
| A mouth | A hand |
|---|
Instructions
Hold your hand about 15cm from your mouth
Purse your lips and blow at your hand
open your mouth and blow on your hand.
Do you notice a difference in temperature?
Now try holding your hand very close to your mouth when blowing with pursed lips or a long way away with an open mouth.
Result
You should find that at 15cm your breath feels cold with pursed lips, but warm with an open mouth.
But if you feel very close with pursed lips it feels warm and cold when you feel a long way away, even with an open mouth.
Explanation
If you create a jet of fast moving air through stationary air it tends to drag the stationary air along with it, essentially due to friction. This is called entrainment, and the effect is stronger the faster the air jet is moving.
When your mouth is open the air coming out will entrain some cold air, but the air which reaches your hand will still be dominated by the warm air from your mouth.
But when your blow hard through pursed lips the jet is moving a lot faster so it entrains more air, and there is less warm air there to start with, so most of what reaches your hand is colder room air. Although this air is slightly warmer than the air in the room it moving so it is much better at removing heat from your hand both by conduction and evaporation so it feels cold.
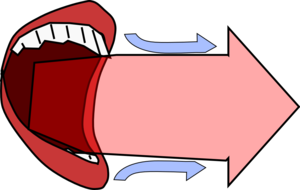 |  |
| With your mouth open most of the air which reaches your hand has come out of your mouth. | With pursed lips lots of cold air is entrained so the air reaching your hand is cold and moving quickly. |
If you feel the air coming out of your pursed lips very close to your mouth it hasn't had time to entrain cold air, so it still feels warm
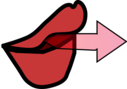 |
| If you feel the air from pursed lips close too it will still feel warm |
And if you feel the air from an open mouth a long way away it will have entrained enough air to feel cold.
asd
- Previous The Potato Arch
- Next How does popping candy work?









Comments
this was a great experiment
this was a great experiment
Thanks for this, I was
Thanks for this, I was thinking about this for months.
Thank u so much
Thank u so much
When you blow air out of your
When you blow air out of your mouth in a large O shape, you feel it as hot. When you purse your lips and you make the volume of your mouth smaller, the air moves from a smaller volume to a bigger volume. Since gas expanded form a smaller to a larger volume, the gas cools down from the 1st law of thermodynamics, and you feel it as cold.
No, that's not the
No, that's not the explanation. It's a good thought, but it's not right. What is written above is the correct answer.
Err, chris, do you mean that
Err, chris, do you mean that Anonymous is incorrect? Actually, Anonymous is correct. He/she is actually more accurate than the explanation provided in the site.
If friction also acts in open
If friction also acts in open mouth case , then why doesn't it acts in tightened mouth case and makes it hotter , in which the friction should be more making air hotter than open mouth case ? Pls. Answer my diellima
Test this hypothesis by
Test this hypothesis by repeating the different mouth blowing techniques in ambient temperatures that are similar to the body's temperature. That is the entrained air stream has an identical temperature to the air leaving the mouth.
Evaporation of the air stream leaving the mouth is also related to the nature of the air flow. The open mouth stream is less turbulent and perhaps even near laminar flow which reduces the evaporation of moisture from the breath air to the surrounding room air.
If you can verify the effect of room temperature on the air entrainment influence on the skin sensation of heat/cold then the effect of evaporative cooing of the air stream cpdriven by turbulence can be determined.
Add a comment