Derek - Hello and this evening we have come to Colchester County High School and with me is Sheena Elliott who set up the experiment we're going to be doing today. This is another easy experiment that you can do at home. Sheena, what are we going to be doing today?
Sheena - We're going to be making matchstick boats.
Derek - Ok, and we've also got to volunteers who've kindly offered to help us with this experiment. So could you please give me your names and what year you're in please?
Zoe - I'm Zoe Taylor and I'm in the upper sixth.
Yichen - I'm Yichen Gau and I'm from the Upper Sixth as well.
Derek - Well thanks for coming along. Are you guys into science? Zoe, what about you?
Zoe - Yep. I do physics and chemistry.
Derek - And yourself Yichen?
Yichen - I just do physics.
Derek - And is this something you're going to carry on doing and you're enthusiastic about?
Yichen - Definitely.
Derek - Well this is great, we've been given some wonderful volunteers here today. So I'll just begin by telling you the things you need to get at home because it's very easy. You need a tray or basin or even your kitchen sink will be fine and you need to put some water in it. You also need some matchsticks, some washing up liquid and finally a knife to cut the matchstick with. We do advise a bit of caution there. Next, Sheena's going to tell us what to do with all these things. Could you explain and perhaps direct Yichen and Zoe about how to set the experiment up here?
Sheena - Firstly what you need to do is take your matchstick and cut it along one end so it looks like a bit of a Y shape. At one end you're just splitting it down the middle so you can open the bits up into the letter Y.
Derek - Ok, is it a bit fiddly work? Is it difficult to do that?
Sheena - All I would say is keep your fingers up the other end of the matchstick when you hold it so you're not anywhere near the knife when you're doing that.
Derek - Ok then. What next?
Sheena - Next we're going to fill up our tray or basin with water. Any depth is fine; just a couple of centimetres or something.
Derek - Ok so Zoe and Yichen do that for us please? We're in the Colchester County High School lab, so go ahead. What now?
Sheena - Now we need to place our matchstick in to the bowl so it's floating horizontally.
Derek - So we're going to be floating it flat and it'll look like a Y shape from above. So Sheena's just dropped it onto the water there and it's floating around very happily.
Sheena - The next thing you need to do is to put a little drop of washing up liquid behind the matchstick. What you're going to be doing is placing it by the side of the matchstick where the Y is opening up; the top of the Y if you like. And you don't want to be touching the matchstick with the washing up detergent. You're just putting it in the water next to it.
Derek - Is it just a drop?
Sheena - Yes, just a drop is fine.
Derek - Ok so you're just dropping a drop of washing up liquid just behind the matchstick, and this means the side of the matchstick where that Y shape has been splayed out. We're not going to do this now of course. We're going to do it later in the show when we reveal to you what the result is. But Zoe and Yichen are hear dying to know what's going to happen. So I'm going to ask them what they think might happen.
Zoe - Maybe the matchstick will move?
Derek - Any idea how or in what way?
Zoe - I don't really know.
Derek - And yourself Yichen? Any idea?
Yichen - I think it will move in some sort of direction.
Derek - Ok well we will see. We also encourage you to try this at home and let us know your result. Until then we will be going back to the studio and coming back to Colchester County High School a bit later on to find out what happens and an explanation from Sheena as well. So until then it's back to the studio.
LATER...
Derek - Hi there and welcome back to Colchester County High School where we do indeed have this experiment ready to go. Sheena has helped us to set up this basin with a layer of water in it and we've got a matchstick that has been cut at the ends so that there's a Y shape at the back of the matchstick. It's floating in the water and we're ready to drop in some washing up liquid. Would you care to instruct Zoe and Yichen about what to do?
Sheena - If you take your washing up liquid and drop a tiny drop just where the Y opens up into the water.
Yichen - Wow! It flew all the way to the end of the tray.
Derek - Ok Zoe, what happened do you think?
Zoe - I don't know but the matchstick moved away from the washing up liquid in the opposite direction.
Derek - yeah that was rather interesting. It kind of propelled it away down the tray. Sheena is grinning gleefully because she obviously knew what was going to happen. Can you explain this for us?
Sheena - This all comes down to surface tension, and so I'm going to explain a little bit about surface tension and what that is. If you imagine that you're a water molecule right in the centre of a tank of water, water molecules have an attractive force between them so you'd be being pulled in every direction by all the water molecules around you. Now if you imagine that you're a water molecule at the surface; you'll be being pulled in every direction from the side and from below but you're not being pulled from the top at all because you've just go air above you there. So what would be the effect on you? Well you might find that you're being squished down a bit and you end up with a more dense film of molecules on top of the water. That's basically what surface tension is. That's why you can get a needle to float on top of the water. It's because the top of the water is more dense. So what's happening with the washing up liquid when we add that? Washing up liquid is something we call a surfactant, and a surfactant is something that breaks this tension. It basically reduces this pulling force of the water molecules. So by adding washing up liquid, one side of the matchstick has the attraction between the water molecules broken, whereas on the other side of the matchstick they're still attracting each other. So what the matchstick feels is a pulling force from all the molecules one side where they're still trying to get closer to each other, but on the other side they're no longer feeling that force. So rather than being repelled by the washing up liquid, it's actually being pulled from the other side.
Derek - So this property of washing up liquid, that it's a surfactant, is this related to its ability to clean things?
Sheena - Yes. It also worked between oil and water as well as water and air. So it's exactly the same thing. It's breaking up this pulling force between materials that are the same.
Derek - So molecules on the surface are pulling in all directions when you lay that matchstick on the surface, but as soon as you add washing up liquid on one side, suddenly the pulling is not there. But there's still pulling from all the other directions and that makes it go in a straight line. Do I have it correct in my head?
Sheena - Yes, that's exactly it. One thing is that if you tried to repeat this experiment, you wouldn't be able to do it without fresh water. That's because a tiny bit of this washing up liquid will pollute your system because you no longer have that surface tension anymore.
Derek - And finally, why is it that we made that Y shape at the end of the matchstick?
Sheena - That just increases the area that you're acting on and gives it a longer bit of match to act on.
Derek - And if people tried it without doing that, what would happen?
Sheena - If your matchstick is at a slight angle, you might find that it spins a bit. It might not shoot forward quite so impressively.
Derek - But still a cool effect and we want you to try it at home if you haven't already. Zoe and Yichen did do it for us so thank you very much. Zoe, what did you think of our experiment?
Zoe - It was really good.
Derek - And Yichen, had you ever heard of surface tension before?
Yichen - Vaguely at school.
Derek - But does this make it a bit more well-explained in your head?
Yichen - Yes it does.
Derek - Great stuff. Well thanks very much for coming along and doing this for us and thanks to Colchester County High School for having us and thank you Sheena for doing the experiment for us. We'll be back next week with some more science that hopefully you can do at home. Until then, it's goodbye from us.
Ingredients
 | A bowl of water |
 | A couple of matches |
 | Some soap or washing up liquid |
Instructions
Fill the bowl with water just a cm or so is fine
Gently float the matchstick on the water.
Place a tiny drop of washing up liquid near the matchstick, it is easiest to do this with another matchstick.
Result
You should see that the matchstick zips away from the washing up liquid as soon as you drop the washing up liquid on the water.
Explanation
This all comes down to surface tension. If you imagine that you're a water molecule right in the centre of a tank of water, water molecules have an attractive force between them so you'd be being pulled in every direction by all the water molecules around you, all these forces would cancel out so you don't move.
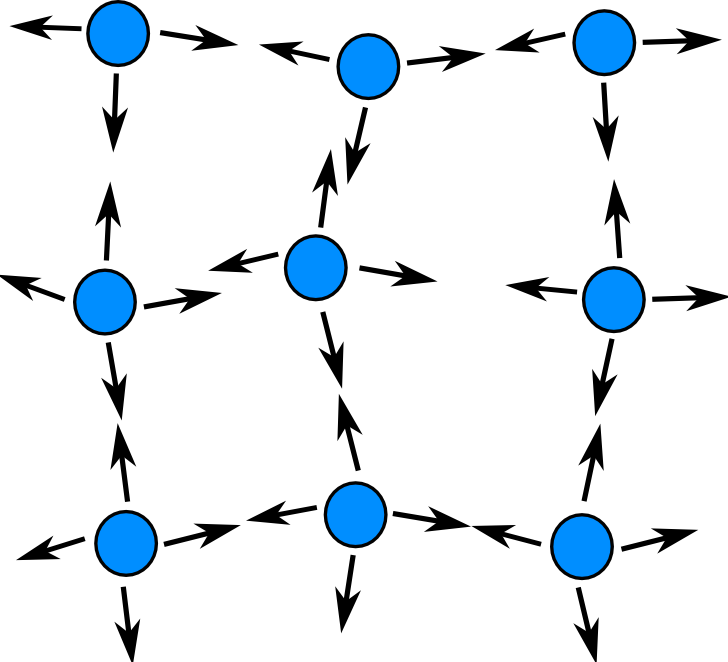
Now if you imagine that you're a water molecule at the surface; you'll be being pulled in every direction from the side and from below but you're not being pulled from the top at all because you've just go air above you there. So what would be the effect on you? Well you might find that you're being squished down a bit and you end up with a more dense film of molecules on top of the water. So water is allways being pulled together, which is why water tends to clump together into droplets.
Water molecules are also attracted to other substances such as wood, glass or paper, so a matchstick for example will be pulled by the water wherever it is touching it.
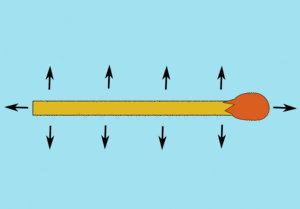
Washing up liquid is a surfactant, which is something that breaks down surface tension. So by adding washing up liquid, one side of the matchstick has the attraction between the water molecules broken, whereas on the other side of the matchstick they're still attracting each other and the matchstick.
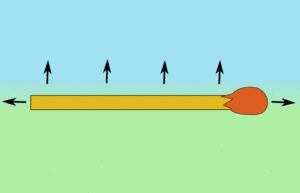
So what the matchstick feels is a pulling force from all the molecules on the clean water side, but on the other side there is virtually no surface tension. So rather than being repelled by the washing up liquid, it's actually being pulled from the other side across the bowl of water.









Comments
Add a comment