Ingredients
A small bottle of unopened fizzy drink (carbonated). It is a good idea to remove the label.
A large bowl of ice - you can bury the bottle in
Water
Salt - several tablespoons
If you have one
A thermometer
Instructions
1- Add some water to the ice so it is about half covered in water
2- Add the salt to the ice (if you have a thermometer this should bring the temperature down to about -4°C
3- Bury the fizzy drink bottle in the ice
4- Wait 20-30mins - if the drink freezes you have waited too long.
5- Open the bottle, watch what happens
Result
The bottle should turn to slush in a few seconds in front of your eyes.
Explanation
Before you open the bottle of lemonade or beer there is lots of carbon-dioxide (CO2) dissolved in the lemonade. If you dissolve carbon-dioxide in water it reduces the freezing point (in the same way that salt does. So you can cool the lemonade down to -2 or -3°C without it freezing.
However when you open the bottle you release the dissolved carbon-dioxide increasing the freezing point and creating lots of little bubbles.
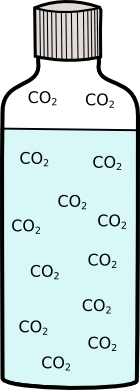 | 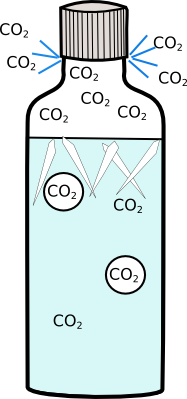 |
Freezing point-3°C | Freezing point-0.5°C |
The lemonade is now below its freezing point, and there are lots of bubbles for the ice crystals to start on, so it freezes in front of your eyes!
- Previous Build a Hot Air Balloon
- Next Seeing the invisible









Comments
Add a comment