Ingredients
 | A glass or a small jug. |  | A torch or the sun on a very clear day. |
 | Some bicarbonate of soda (baking soda) |  | Some vinegar |
Instructions
In a reasonably still room:
Put 2-3 teaspoons of bicarbonate of soda into the glass
Add vinegar, until the glass has been filled with foam
Wait for the foam to die down
Turn on the torch
Carefully put the glass beween it and a white wall - or if it is a sunny day in front of a sunlit white wall will work too as long as you are inside.
Imagine the glass is full of a fluid and pour that fluid out.
Look at the wall as you are doing this.
Result
You should see very faint shimmery patterns pouring out of the glass, even though you can't see anything there.
Explanation
There is a gas called carbon-dioxide locked up in the bicarbonate of soda. When you add the vinegar you will release forming lots of bubbles and foam. When the foam dies away this gas sits in the cup because it is heavier than air.
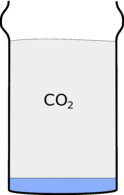 | 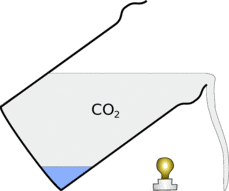 |
This also means that when you pour the carbon-dioxide out of the glass it will fall down in a stream.
Light travels slightly slower in carbon-dioxide than it does in normal air which means that the light gets bent if it hits the carbon-dioxide at an angle (see the
Pyrex in Oil experiment)
This means that in some places light from a few areas of the stream is bent together, focussing it and making parts of the paper brighter. There will also be shadows where the light would have gone if it hadn't been bent somewhere else.
If you put a glass up to the light you will see a similar effect. These patterns are in the general shape of the carbon-dioxide allowing you to see the otherwise invisible gas.
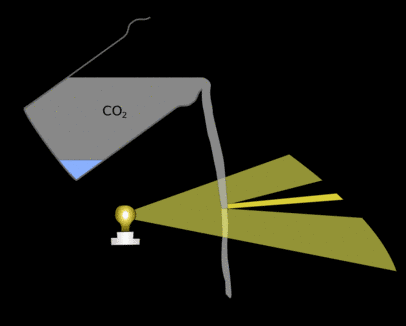 |
| Light is bent when it hits the stream of carbon-dioxide it is concentrated in some places making other areas darker. These areas of light and dark are what you can see on the paper. |
- Previous Freezing lemonade bottles
- Next Custard Fireballs









Comments
Add a comment