Bones contain a goo-based shock-absorber
Interview with
Earlier this week scientists in Cambridge revealed that the minerals that make up our 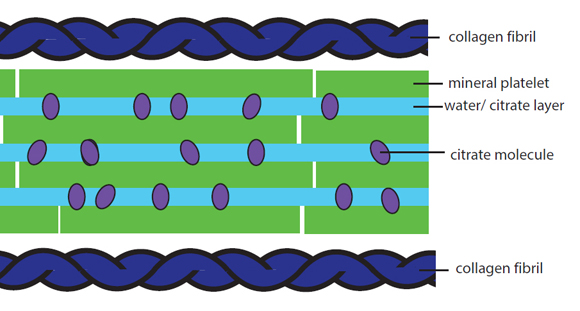 bones actually largely consist of a so-called 'goo', which works as a shock absorber.
bones actually largely consist of a so-called 'goo', which works as a shock absorber.
Melinda Duer, from Cambridge University and led the study, explained the findings to Harriet Johnson...
Harriet - Hi, Melinda. So, what is this goo and why is it so important?
Melinda - Okay, so what we knew about bone mineral before we started this study is that the mineral consists of these really tiny, tiny nanoscopic plates of calcium phosphate. They stick together in stacks between these ropes of collagen protein in our bones. So, that's what we knew. What we didn't know was what held those stacks together. Now, we had an inkling it had to be something plastic or flexible, because it wasn't frankly a stack of little plates like that. It's just brittle. So, that was what we knew. It took us 4 years to work out what was between those plates. But when we finally worked it out, it made complete sense because what's there is "goo" and it's a goo of citrate and water, and some of the calcium phosphate mineral ions as well in there.
Harriet - So, why did it take so long to find out this structure?
Melinda - Good question and it wasn't just because we were being lazy. So, if you're looking at what we call an ordered structure, crystallised structure are a regular ray of atoms and molecules. There's quite a lot of techniques that can help you find out about that. They're well understood. When you're dealing with something where the atoms and molecules are disordered, they're just in some kind of almost random arrangement. It's really tricky to find anything that can help you find any clues about that. So, what we had to do is we use lots and lots of different techniques and we pieced together the information. A little bit of evidence from here and there. Our team at the Advanced Imaging Centre in Cambridge came up with some things, our nuclear magnetic resonance spectroscopy came up with others, and then our fantastic theoretical physicists at UCL Chris Pickard was able to help us put those ideas together into a structural model. We can then do some calculations on, see what kind of experimental event should be coming out of that model and we go back and check, and look for that. Now, we had to go through an awful lot of models before we started to say something that might actually be reality.
Harriet - So, a big project. So, this is believed to be a root cause of osteoporosis and other brittle bone problems. How is that and how can we use this information to improve these conditions?
Melinda - On the macroscopic level, there's going to be a lot of reasons why bones are brittle, but if you drill down to the molecular level, there's a more restricted set of reasons why those molecules either have the wrong structure or the wrong interactions with each other. And so, that's what we're looking at here. Essentially, the citrate and water goo allows the mineral plates to slide over each other. So, as you jump up and down your bones, your bones flex; those mineral plates can actually move and they're not going to be damaged by that impact. But if that goo is not there, then what you got left with are these crystals, these flat plates stuck together and more of this big brittle lump and they're going to be fracturing. They're just a very brittle mass. So, the question is, why does this citrate get in there and why does it sometimes not? Our hypothesis - and this is just what it is at the moment - is it comes down to how this mineral is formed. That's formed - as I've said before - in between these ropes of collagen matrix and those spaces where it forms are really, really small. The citrate delivers calcium to that space to make the calcium phosphate and then it gets trapped. It's a very small space. The minerals are forming. The citrate can't get out. What we think happens when you have osteoporosis or brittle bone disease is that the space the mineral is forming is just much larger. So now, you know forming the mineral inside a calf and of course, the citrate can then get away. It doesn't have to be incorporated. So you end up with large blocks of mineral without citrate and hence, brittle.
Harriet - So, this essential goo is leaking out of the bones and then causing these problems. Thanks to Melinda Duer from Cambridge University. She published that work in PNAS this week.
References
- Previous Anti-aphrodisiacs, for flies
- Next Spin and antimatter









Comments
Add a comment