This Week in Science History - Mendeleev's Periodic Table
Interview with
This week in science history saw, in 1869, Dmitri Mendeleev presented his theory for the ordering of the elements into a periodic table to the Russian Chemical Society.
 In 1869 houses were still lit by gas lamps, the Suez canal opened, linking Europe and Asia by water, without having to navigate around Africa, and the first issue of the journal Nature was published.
In 1869 houses were still lit by gas lamps, the Suez canal opened, linking Europe and Asia by water, without having to navigate around Africa, and the first issue of the journal Nature was published.
The first to suggest that there were more elements in the world than the four of earth, air, fire and water, as suggested by the Greeks, was Robert Boyle in the mid 1600s - over 200 years before Mendeleev presented his table.
Several other scientists in the 19th Century had attempted groupings of elements, such as Johan Dobereiner, whose Law of Triads of 1829, suggested that the elements should be grouped in threes where the middle element would have properties that were the average of the elements either side. His example of chlorine-bromine-iodine was extended some years later to include fluorine, and other scientists realised properties extended over groups larger than three. As more elements were discovered, patterns in their properties began to emerge.
The French scientist De Chancourtois and the Englishman John Newlands both put forward more complex ideas on the ordering of elements in the early 1860s, but by 1869, 63 elements had been discovered, and Mendeleev chose to order them in a way noone had done before.
 He wrote out a card for each element, with their name, atomic mass and chemical properties on, then laid them out like a game of solitaire, according to their chemical properties, rather than their mass number, which despite being pretty inaccurately calculated, was the conventional way to set them out at the time.
He wrote out a card for each element, with their name, atomic mass and chemical properties on, then laid them out like a game of solitaire, according to their chemical properties, rather than their mass number, which despite being pretty inaccurately calculated, was the conventional way to set them out at the time.
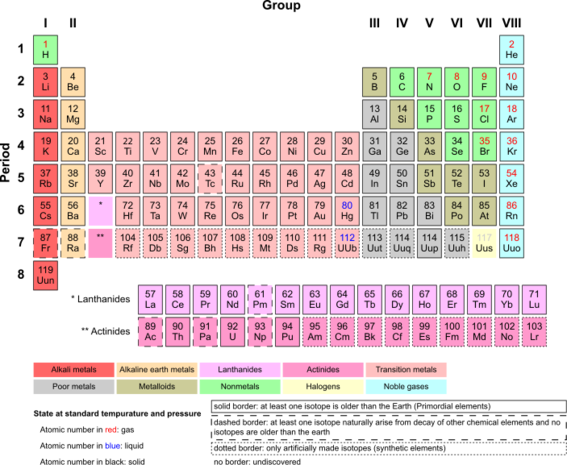
Mendeleev's Periodic Table showed the similarities and trends over much larger numbers of elements, both in vertical groups and horizontal periods in the table, rather than just in small groups such as in Doberiener's Law of Triads. He left spaces for elements he believed were yet to be discovered, and predicted their properties. He was proved right by the discovery of gallium, scandium and germanium later in the 1800s, and it is because of these predictions that Mendeleev is often considered more important in the discovery of the periodic table than the German scientist Lothar Meyer, who independently published a very similar table just months after Mendeleev.
In the 20th century, the table has been expanded and refined, to include elements that have been synthesised or are the result of nuclear decay rather than just those occurring naturally, but it still follows the same basic layout that Mendeleev predicted.
Knowing the order of the elements is not just a great teaching tool to understand how and why chemical properties change down a group or across a period, but also has had applications in industry, allowing people to predict how different compounds will respond when reacted.
- Previous Self-flying planes and Water Batteries
- Next First Fish Sex









Comments
Add a comment