Improving Enzymes
Interview with
Chris - One of the main aims that scientists have for synthetic biology is - rather than relying on nature to come up with all the answers, instead, we want to be able to take what nature has already made and make it even better for doing certain jobs, and Dr. Ross Anderson at Bristol University is trying to do just that.
Ross - Essentially, what I want to do is create an enzyme from scratch. So enzymes are a class of protein. Proteins, being a polymer of molecules we call amino acids. They're protein catalysts. They perform and accelerate chemical reactions and there's a huge diversity of enzymatic catalysis, so photosynthesis for instance, hydrogen production. There's a huge range of reactions which would be exceptionally good for us to actually tap into and harness the power of like solar power to generate hydrogen for instance. That'll be very, very attractive.
Chris - So these are nature's catalysts...
Ross - Yeah.
Chris - ...without them, the chemical reactions that keep us alive just couldn't happen. It sounds like nature's doing a wonderful job. Why do we need you? I mean that in the nicest possible way.
Ross - We still, after all these years working with proteins, have a fairly tenuous understanding of how to build function into them. So we have a relatively decent understanding of how enzymes work, but so far, we've been unable to actually make one from scratch. I think that there's a big gap in our understanding - if we can't make something from scratch, how do we truly understand it? So that's really where I'm coming from and I think that if we can harness nature's tool kit then there's a whole variety of possibilities available to us. The other thing is that through evolution, how these proteins have actually evolved and have been put together by nature, we have to some degree lost that history, and we don't have the benefit of seeing the selective pressures that these proteins were under through the course of evolution.
Chris - In other words, we're seeing the finished product rather than the product being developed and that makes it much harder to get to the nub of how these things, these micro machines, work in the first place.
Ross - Exactly. So we see a protein that's been evolving for several billion years and then we want to change it in some way to match our own needs, and the problem we have is that it would be like, for instance, taking a Ferrari and trying to make it into a bus. The essential elements are the same; an engine, wheels, but you know, it's going to be quite complicated to approach that in that particular way.
Chris - So go on, give us some examples of things you've been working on.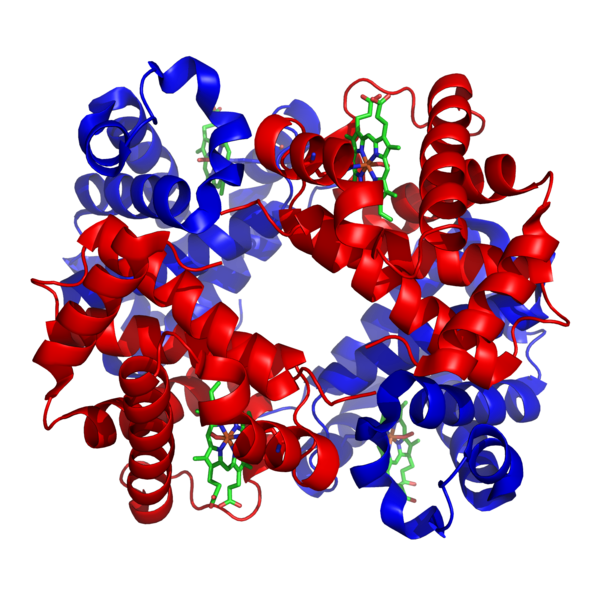
Ross - So an example is an oxygen binding protein. We've called it an artificial myoglobin or neuroglobin and what we did there was start with a very generic sequence, so we started with a protein that was made up of 100 amino acids, but it was only three different amino acids that made up these 100. So we know it folds into a particular structure which is useful for us and then we sequentially added functions, so we took a molecule which is present in haemoglobin and myoglobin. It's called haem and we inserted that into the protein interior. And then what we did after that was change the sequence of the protein so that oxygen could actually access the haem molecule and reversibly bind. This was actually the first example of a protein built from scratch that could do this particular function.
Chris - Is this all done in vitro? In other words, you make these things artificially in a dish, or are you actually at the stage where you can say, "Well that's the protein I want so I can work out what the gene sequence would be and then I can make that gene sequence and say, put it into E. coli or yeast or something to get that to make it for you."
Ross - It's a little bit of both at the moment. What usually happens is that we start with a protein sequence that we quite like and then we would synthesise it in vitro. There's fairly decent systems now that are kind of almost like a robot which will build up your protein sequence for you. They're quite expensive though and so we tend to prefer ordering an artificial gene which are now very, very cheap. Then we get something like E. coli or as you say, yeast to make the protein for us. So, generally, the proteins I work with, I get E. coli to make them which ends up exceptionally cheap in the end.
Chris - Are there any risks associated with doing this kind of thing?
Ross - With my work, I would say no. the gene that we insert into E. coli is completely benign and certainly, it doesn't change how toxic the E. coli is to us. So in that sense, no, there really are no risks with what I do personally.
Chris - And looking to the future, say we can build bespoke proteins, would there be both therapeutic and industrial applications here? Could you take some of these diseases that people suffer from because one of their own proteins is the wrong shape and build one that works better for them and put it in?
Ross - Yeah, absolutely. This is really what's going to happen in the future. We're really at the beginning of this whole field even though it's been running for about 10 - 15 years. There's not been a huge amount of progress. Primarily, just because there's not really that many groups who've been working in it. But what we could definitely see in the future is targeted therapies for improperly folded proteins in the body. Also, directed therapies against cancer for instance, we can make antibody silent proteins to go in and perform a reaction which kills cancerous cells for instance. Industry is really where my work would be more applicable to. In the future, in the absence of crude oil, we're going to have to look for other sources of fuel and a big area of interest is making methanol from methane. Again, nature does this fairly well in the bug, but when we try and work with the proteins outside the particular bug, they're not so amenable to industrial processes. So what we're really trying to strive for is a cheap form of catalyst that we can just grow, essentially.









Comments
Add a comment