The Art of Alloying
Interview with
Diana - We humans have been moulding metal to our will for at least 3,000 years. In fact, our use of metals mark the beginning of the aptly named Bronze Age. These days we fine tune the properties of metals to perform in extreme circumstances - inside nuclear reactors, jet engines, rockets, et cetera. To find the right combination of properties, we rely on metallurgists like Howard Stone at Cambridge University's Department of Materials Science and Metallurgy.
Howard - Well one of the first things I don't think people often get much an appreciation for is the fact that metals are actually crystalline materials. In other words, if you look at them on a very microscopic scale, at the atomic scale, you will see that the individual atoms are arranged in a very precisely defined periodic array or pattern. What you have to imagine if you were looking at your piece of cutlery or the tip of your ballpoint or even that legs of your chair is that the metal itself is built up of all these little individual crystallites, exactly or analogous to grains of sand on a beach or sugar in a bag of sugar. Only that there was no space between the grains. It's been, if you like, pressed together so that there's no distance between these little individual crystallites.
Diana - But not all of the crystals in any given metal will be the same.
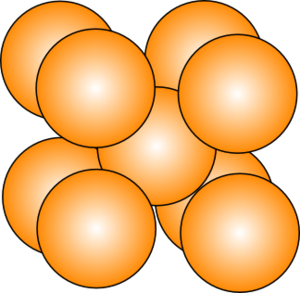 |
| Body-centred cubic structure |
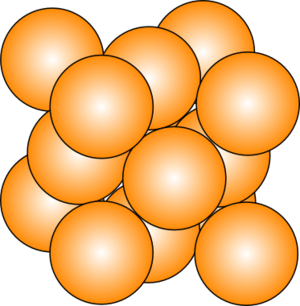 |
| Face-centred cubic structure |
Howard - The individual crystal structure, the different arrangements that can be adopted by these metals, have a profound influence upon the properties of the materials. And the presence of these phases as we refer to them - in other words regions of material which have these different crystal structures - does have a very important influence on the properties of metals themselves. It's our role as metallurgists to work out what the best possible combination of phases we can get out of a material is and therefore, the best possible set of properties that we can possibly achieve.
Diana - One way to alter the crystal structure of a pure metal is to add other elements. Steel for example is a mixture of iron and a small amount of carbon, but its properties are surprisingly different from both original materials.
Howard - If we add any other element to any given element, metallic element, so if we for example add carbon to iron, then we might expect to form new phases. In steels, we can expect a whole series of carbides as a possibility, and the morphology and crystal structure of those individual carbides can be very different, depending upon the conditions in which it's been processed and also the process of other alloying elements, and that will have a very profound effect upon the properties of the resulting material.
Diana - These mixed metals or alloys may be able to perform a given task far better than the pure metals and as such, are found all around us.
Howard - Well if you look at your average piece of tableware cutlery, you're probably looking at a steel, so an iron based alloy, which contains significant quantities of principally chromium and nickel. If you look at an aeroplane for example, most of the air frame will be made of aluminium alloys. The engines themselves may very well contain far more exotic things like titanium and nickel alloys as well.
Diana - For extreme environments like the high temperature high torque conditions inside a jet engine, we turn to a subset of alloys called the superalloys.
Howard - Nickel based superalloys justifiably deserve their name because they're the only class of materials that are able to operate at a combination of very high temperatures and very high stresses, whilst maintaining their surface stability. i.e. not being degraded too badly by the environment in which they operate.
Diana - But what is it that makes these particular alloys so good?
Howard - They're based principally on a combination of nickel and aluminium. So nickel is an element which adopts a face centred cubic structure. In other words, if you looked at the atomic scale, you'll see the atoms arranged in little cubes with the atoms on the corners of those cubes and then one in the middle of each face. Now with the addition of aluminium, the alloy will form precipitates, in other words, an additional phase which comes out of the material, embedded within the material, which are Ni3Al. In other words, for every 3 nickel atoms, you'll find another aluminium atom and they adopt a very precise crystallographic arrangement relative to each other. If you imagine my little cube again, I will have aluminium atoms in the corner of the cube and now nickel atoms in the face of each cube. Because of the similarity of that particular crystal structure with the nickel itself, these precipitates are able to form in very good registry with the matrix in which they form, and this creates a real barrier to motion of atoms past each other through the crystal structure and really resists deformation at very high temperatures.
Diana - Making the most of superalloys can involve adding small amounts of very exotic metals which can improve the properties but as is the case with exotic things, this comes as a cost.
Howard - Well over the last 50 years, we've done everything we can as metallurgists to improve the properties of nickel based super alloys by considering all the possible alloying elements we can reasonably include. Commercial nickel based super alloys for turbine blade applications for example, now can contain 9, 10 or even more elements in very precisely controlled quantities to get optimal properties. Some of the latest elements we've considered including include things like ruthenium which is a very rare platinum group metal. And that has extended the temperature capability that super alloys are now able to operate beyond those of previous generations. But these are very rare, very expensive elements. The real challenge for us is to identify whether there are any other alternatives to nickel based super alloys and that is a challenge which many people have been investigating over the last 50 years.
Diana - That's Howard Stone, assistant director of research at the Department of Materials Science and Metallurgy at Cambridge University.









Comments
Add a comment