Treating Respiratory Disorders with Gene Therapy
Interview with
Maria - My interest has been in cystic fibrosis (B) but more recently I have started to work in gene therapy for eye diseases.
Chris - Can you just review for us what we understand about cystic fibrosis - what it is, what causes it, why it happens.
Maria - So cystic fibrosis is a very devastating disease. It affects Caucasian populations and the frequency is really high for Caucasian populations, so 1 in 2,000 live births is affected with CF. There's no cure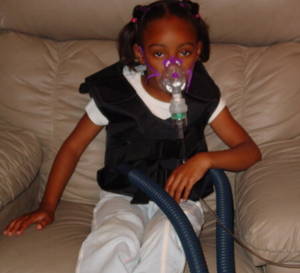 for the disease and for someone to imagine the impact of the devastation of lung disease, you just think to your worst cold ever, your worst chest cold ever, and that progressively getting worse. Patients basically don't have treatment and when lung disease becomes so bad, the only option is a lung transplant and of course, lung transplants...
for the disease and for someone to imagine the impact of the devastation of lung disease, you just think to your worst cold ever, your worst chest cold ever, and that progressively getting worse. Patients basically don't have treatment and when lung disease becomes so bad, the only option is a lung transplant and of course, lung transplants...
Chris - There are not many of those going around, are there?
Maria - Exactly, lung transplantations are down. So it's a very poor outcome for patients with severe lung disease.
Chris - At a molecular level, what do we understand about what's causing the condition?
Maria - So the gene was identified almost 20 years ago, 22 years ago to be exact, and it's a gene that encodes a chloride channel. The chloride channel has got a very fancy name - the cystic fibrosis transmembrane conductance regulator (CFTR)and it's situated on the top of the epithelial cells which are cells that line the lungs. And the function of this channel is to control the fluid that lines our lungs which allows us not to get colds when we walk into environments that are either dusty or full of bacteria and viruses.
So, the first thing that you do when you walk into a dusty room is cough and that's the natural defence of your lung to get rid of what it's inhaled. In the case of cystic fibrosis where those patients don't have that gene and the product, the lungs don't have the normal function of actually trying to get rid of all those noxious particles that they inhale. Which means that everything that they inhale remains in the lungs and whether it's bacteria or viruses, they replicate, they make a really nice home for themselves and progressively worsen the lung disease.
Chris - I'm quite glad you brought up the idea of how lungs defend themselves normally because that must be a big challenge for a gene therapist to need to overcome. Lungs are pretty good at preventing themselves from getting infected because they have all these defences - mucous and they have this mucous ciliary escalator that washes the mucous out - so anything you want to try and get into a lung cell has got to bypass all those defences first.
Maria - Exactly, exactly right. So, the same way that our lungs protect us from those daily encounters with the bacteria and the viruses, they don't have the ability to know the difference of a gene therapy vector versus something noxious that they've inhaled. So the same defences that apply to those noxious particles will apply to a gene therapy vector. So what we need to do when we develop the gene therapy vectors, and this is work that we've constantly been doing in the field is develop vectors that actually can bypass those barriers, and if we can't find vectors that can bypass those barriers, we try to momentarily disrupt those barriers.
Again, those barriers are there for a reason and that's to protect us from noxious particles, we don't want to introduce them along with the gene therapy vectors in those patients that will be susceptible to infections.
Chris - Some people are trying to persuade the lung cells to take up individual bits of DNA. Others are using viruses to do this. So is that your approach?
Maria - Yes. So we're using viruses. We believe that vectors that are based on viruses are very effective. We've all had our common cold and we've had it more than once and a common cold is very effective, you see the symptoms straight away, and it spreads throughout the lung very quickly. So we're trying to harness that ability of a virus to get into the cells of the epithelium, the cells of the lung to actually use that.
Chris - Which viruses are you using?
Maria - So we're using a small virus. It's called an adeno-associated virus. It's never been associated with any human disease. It's effectively been used and translated into clinical trials and in fact, there have been recent successes with eye diseases using this particular virus.
Chris - So how are you using it - what do you need to do to that virus so that you can get genes into the lung cells that need them?
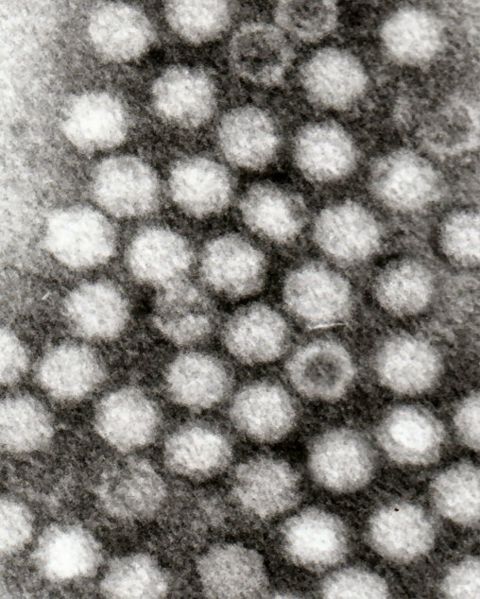 Maria - So in terms of the virus, what you do is you sort of remove what you consider the virus to have in terms of pathogenic genes. So you remove those genes that are pathogenic that could potentially cause disease and you replace them with a therapeutic gene - in this case, cystic fibrosis gene - but you allow those genes that are basically necessary for that virus to attach onto the cell, to move through the cell, to go and integrate, or stay close to the DNA of the host cell.
Maria - So in terms of the virus, what you do is you sort of remove what you consider the virus to have in terms of pathogenic genes. So you remove those genes that are pathogenic that could potentially cause disease and you replace them with a therapeutic gene - in this case, cystic fibrosis gene - but you allow those genes that are basically necessary for that virus to attach onto the cell, to move through the cell, to go and integrate, or stay close to the DNA of the host cell.
So, we try to harness what's good about the virus and remove what's bad about the virus - remove the bad part of the virus and incorporate the therapeutic gene. And so, in essence, the same way that you would get your common cold and all those symptoms, you will now replace the common cold with the cystic fibrosis gene.
Chris - So the virus that came in from the cold if you will. The problem I can see with this though is that the cells that you're going to hit with your therapy because this is something a patient is going to breathe in or have washed into their lungs isn't it - you're going to hit cells that are short-lived.
Maria - Correct.
Chris - And that means that you're going to have to keep repeating this therapy. So I can see sort of two problems - one, that's an inconvenience and two, won't the patients become immune to continual exposure to this virus and they'll just develop antibodies and that will prevent you doing this?
Maria - Correct. So, depending on the virus that you use, it has the ability to stably integrate into the genome, into the DNA of that cell. Now in the case of - for example, a ciliated cell which is the cell that actually lies on the surface of your lung - that's considered a terminally differentiated cell which means that once that cell dies, it does not give rise to any gene-corrected cells. We still don't know, even though there are hints at what is the actual stem cell, the progenitor of the airway, but with the viruses that we have in hand, we don't only target one specific cell type. That's the beauty of the viruses that we use, that we can target several different types of the airway epithelium. Now ideally, you'd like to target the stem cells.
We don't know which ones those are, but we're hoping that with the strategies that we're using, at least one population or subpopulation will have the ability to become a stem cell or to give rise to gene corrected cells. Now in the event that you don't transduce or target those stem cells, you will need to re-administer your virus. Now, we do have some window, some time in terms of getting a re-administration. These cells live between 3 and 6 months, so with certain viruses, the neutralising antibody comes down, to a low level, that will allow re-administration of the virus.
Chris - You can get around it just because the immune system loses interest.
Maria - Exactly!
Chris - And you can come back in and hit it again.
Maria - Exactly!
Chris - Wouldn't a better way though when you sort of hinted at this with the stem cell idea - wouldn't a better way be to inject the virus into the bloodstream and program it to go for the stem cells in the lung, assuming we can find them? So that you would hit the stem cell, and then it would propagate into all of the daughter cells the beneficial change that you've impregnated it with effectively?
Maria - Yes, that could be an option. The problem is that once you inject things into the systemic circulation if you will, everything gets cleared by the liver. And so, you'll either end up losing a lot of your virus that's swept by the liver, or you'll have to inject bucket loads of vector. Now the more vector you inject into a human, the more likely it is that you're going to have a devastating immune response just by the mere fact that you're injecting a lot more vector that you need. So while that could potentially be an approach, a more effective way is to actually try to localise your delivery to where you want the vector to actually transfer the gene of interest.
Chris - And to finish off Maria, how close are you to being able to do this in a human.
Maria - In terms of the work that we do in our lab, we focus on adeno associated viruses. We have collaborations with others that work on Lenti viruses. The field has made tremendous work and advances over the last 10 years. We're not ready for a clinical trial. We're still answering questions that have become apparent as a result of clinical trials that have not produced the results that we expected, and what that allowed us to do, if you will - the failures - even though that's potentially a wrong word for a clinical trial, the failures that became apparent allowed us to ask questions that have become very important as we try to move forward with gene therapy, which is trying to make our vector more efficient, trying to make a vector more safe.
And the truth of the matter is that you don't really know how well your vector is going to be unless you inject it in a human, and that's the case that's happened with many clinical trials, and the experiment actually starts when you inject to human. In terms of the progress in the field so far, there is an active gene therapy trial and that's the one from the UK Cystic Fibrosis Gene Therapy Consortium in which they're using a lipid-based approach to actually deliver plasmid and coding for the cystic fibrosis gene in patients with cystic fibrosis here in the UK.









Comments
Add a comment