Graphene is the focus of this week's Naked Scientists, including how it holds the key to the super-flexible touch screen displays of tomorrow, super-light composites and the next generation of computer chips. In the news, a breakthrough in understanding Alzheimer's Disease, why glider pilots should be paying more attention to how falcons fly and why a new exoplanet has led astronomers to question current theories of planetary formation. Plus, we celebrate the first chunk of cheese to make it into orbit and ask if there's any evidence of a health benefit from wearing magnetic bracelets...
In this episode

01:57 - New High Density Ultracapacitors
New High Density Ultracapacitors
There are two main traditional ways of storing electrical energy, you can use a rechargeable battery to store the energy chemically, which will store quite a lot of energy up to 350 watt hours per kilogram, but it will do so quite slowly and for a limited number of recharge cycles. The other option is to use a capacitor, this in a simple case is basically two sheets of metal with a layer of insulator in between. If you apply a voltage some charge will flow onto the sheets and will be attracted to the opposite charge on the other sheet. because they are not using chemical reactions capacitors can charge and discharge thousands of times faster than batteries and they waste much less energy as heat. 
The problem is that conventional capacitors store less than a thousandth of the energy of batteries. To increase this you need to reduce the distance between the plates stabilising the charges and allowing the capacitor to store more energy at the same voltage. Recently by using activated carbon with a huge surface area filled with an ionic liquid as an electrolyte supercapacitors have got this up to and energy density about as good as lead acid batteries.
Now Chenguang Liu and colleagues has used graphene, which is essentially a single layer of graphite a single atom thick as the electrode. This is about the thinnest an electrode can get, and they have altered the graphite so that it is curved, and won't lie flat and stack. This means that the ionic liquid electrolyte can get between the layers and produces a very efficient capacitor. They can store between 80 and 136 Whrs/kg which is about as good as a nickle metal hydride battery, which were the standard batteries 10-15 years ago. That might not sound very impressive until you realise that it would charge in seconds or at the most a couple of minutes as opposed to hours for a lithium ion battery.
This would both allow an electric car to charge up as fast as you would normally fill up with petrol, and make it ideal for storing large amounts of energy very quickly such as when you brake hard in a hybrid vehicle.

04:29 - An Insect’s Eye View of the World
An Insect’s Eye View of the World
A new database named FReD could help to establish how bees see flowers, helping researchers understand more about bees, one of the world's most important pollinators...
Bee vision is very different from human vision, they perceive colours we are simply incapable of seeing.
Most insects have light sensitive cells, or photoreceptors, that are sensitive to ultraviolet, blue and green light and many have four or more receptor types, allowing them to perceive a wide range of wavelengths of light from long to very short. In comparison, human eyes are just not up to the job of assessing flower appearance objectively.
Now, for the first time, a database has been developed that collects an extensive range of data on the full reflectance spectrum of flowers, and is freely available online.
Publishing in the open-access journal PLoS One, Sarah Arnold from Queen Mary University of London and colleagues announced the creation of FReD, the Floral Reflectance Database, which contains the reflectance spectrum, or how much light is reflected at different wavelengths, for flowers from all over the world.
To really understand the environment as perceived by an insect you also need to know which wavelengths the species can detect. This is also included in FReD and allows researchers to create a "colour space" for an insect, observing flowers specifically from that species' perspective.
FReD is set to grow as its users provide more reflectance data from different species and habitats and, although the priority so far has been bees, improve the catalogue of insects available.
As FReD contains data on flowers of different ages but from the same species, it can also be used by botanists researching global trends in flower colour, plant growth and development; by ecologists studying habitat diversity and interactions; as well as those researching the vital role that pollinators play in our environment.

07:18 - What Causes Alzheimer's Disease?
What Causes Alzheimer's Disease?
with Randall Bateman, Washington University School of Medicine
Ben - Alzheimer's disease is a common cause of cognitive decline amongst elderly people. An estimated one person in every five over the age of 80 is affected, and develops a range of symptoms including memory loss. The cause of the disease is a build up in the brain of a protein called beta amyloid which damages nerve cells, but why does this happen? Chris Smith spoke to Randy Bateman, a neurologist at Washington University in St. Louis...
Randy - So, there was a basic question which we began to consider about four or five years ago, and the question was, what causes Alzheimer's disease? And that question has been asked for a long while, but this was more directed at the current thinking about what causes Alzheimer's disease in relationship to what's known scientifically. There's an amyloid hypothesis which specifies that build up of a protein called amyloid beta, which is normally made by our brains, that this increase in this protein in the brain leads to damage that causes the symptoms of Alzheimer's disease. Now the basic question that we were asking was in Alzheimer's disease, does the amyloid beta build up there because it's being produced too much, or made too much in the brain, or is it there because once made, the brain has a problem clearing it away? Some of the basic information about amyloid beta is that the brain normally makes this, the neurons or the thinking cells of the brain normally produce this amyloid beta protein in the brain, and it's normally cleared away and it doesn't build up into very high amounts. But what we also know is that in patients that have Alzheimer's disease, their brains are filled and littered with this substance up to 100 to 1,000 fold normal levels. So it builds up at very high levels and this is thought to be toxic to the brain and cause damage, ultimately culminating in dementia of the Alzheimer's type.
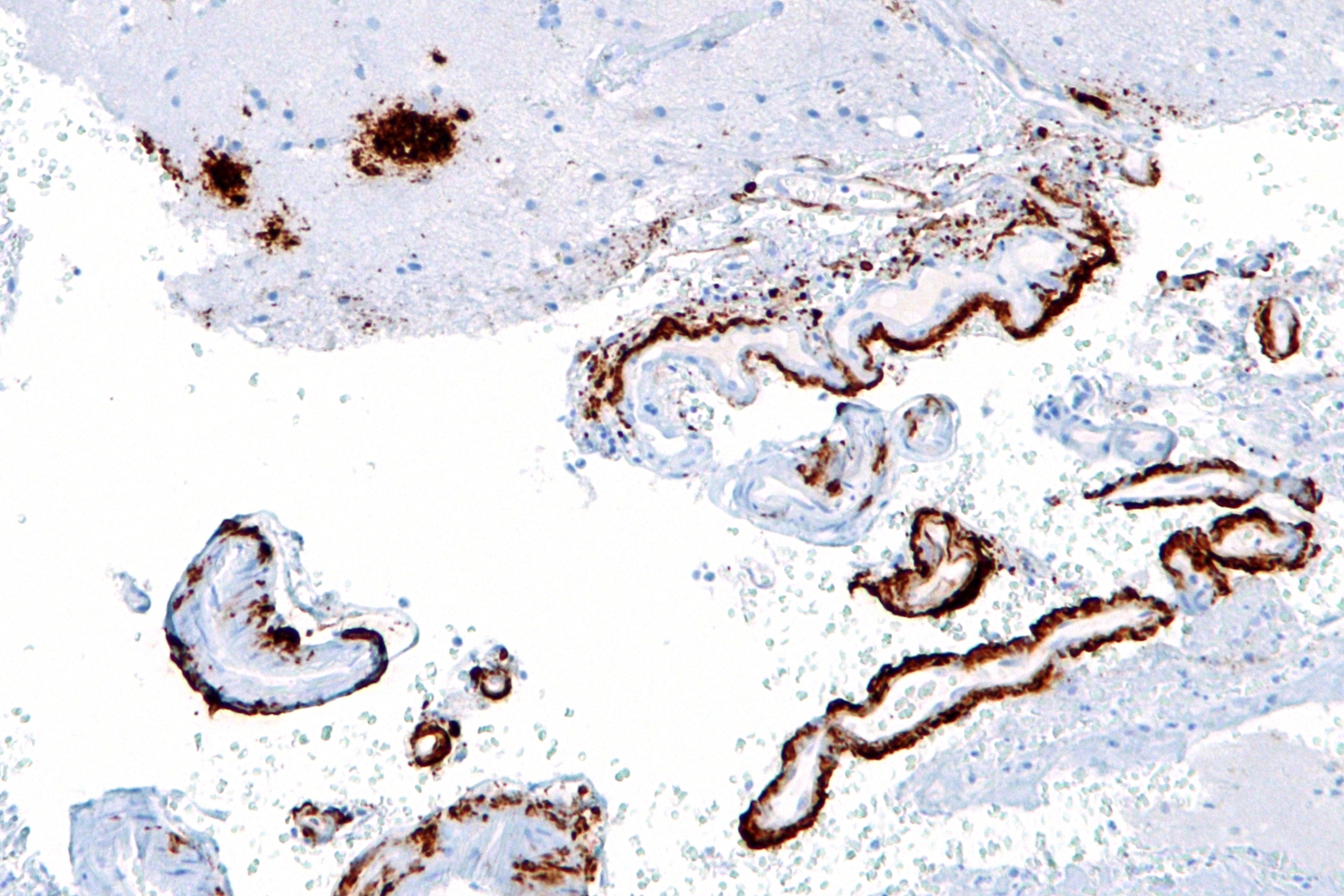 |
Micrograph showing amyloid beta (brown) in senile plaques of the cerebral cortex (upper left of image) and cerebral blood vessels (right of image) with immunostaining. © Nephron @ Wikipedia |
Chris - So you've got two problems there. One of them is, that there's too much being produced or is it that it's not being dispensed with, dealt with by the brain as it is in a healthy person's brain? So how can you try and disentangle those two?
Randy - That was the challenge and that's where the technique was actually developed to answer this question. Essentially, the way to do this is to label the proteins as they're being made so you can track them. And then once a protein is labelled then you can track how fast it's being produced, how quickly it's being cleared away. The first publication of the method itself was in 2006 where we reported what normally happens in young healthy people with amyloid beta in the brains in terms of its production and its clearance rate. And often, I use an analogy that this labelling system - imagine that you have a single water and you have a faucet on with water coming into the sink, and you have a drain that's draining the sink at the same time. If you just look at the sink and you don't look at the faucet or the drain, you only see a level of water in the sink, and that's what we normally measure when we sample the fluid that surrounds the brain. We measure how much of that amyloid beta protein, or the water level, is there. One way to track how fast it's coming in is, you can imagine that if you dyed the water coming out of the faucet a certain colour, say blue, that the water in the sink would turn bluer and bluer over time, the more blue water came into it. And if you watch over time, how much of that water has been labelled with that - in this case, a colour label. In the case of the amyloid beta protein, we're using a label that's just slightly heavier for the protein then we can estimate how fast the production or the rate of water coming in to the sink is. And by the same token, if you stop labelling and then you watch the water as it's cleared away, new fresh clear water comes in and the blue is cleared away, you then have an estimation of how fast the sink is draining that water. And so, this is what we do. We take a label that incorporates into the protein, it's an amino acid which is a building block of proteins which our body normally makes, and it's slightly heavier than normal amino acids. We infuse that into the bloodstream of the person and all the proteins that person is making can be tagged with this label, marking it as newly made. Then we sample over time the fluid that surrounds the brain, it's called cerebrospinal fluid and we watch the appearance of this newly labelled amyloid beta protein, and then we stop labelling and we watch how that labelled amyloid beta is cleared away over time.
Chris - And you did this in healthy people and you did this presumably in a group of Alzheimer's patients to compare the rate of production and removal of the beta amyloid in the brains of both?
Randy - Exactly. And so, in this study, what we did is we compared 12 people who have Alzheimer's disease to 12 people who don't have Alzheimer's disease, but are about the same age, and we compared the two to find that there was a significant decrease in the clearance of amyloid beta. But on average, there was no significant change in the production rate of amyloid beta.
Chris - The only problem with this study is that it tells you about people who've already been diagnosed and it would be very interesting to wind the clock back in their lives, or look upstream - look in people who are going to develop Alzheimer's disease or just look in people who are viewed as healthy, and then follow them up, and then see who does get Alzheimer's disease and see if there's anything lurking upstream in those people.
 |
Alois Alzheimer |
Randy - That's exactly right. That's an insightful point and one which we're planning on doing as the study goes on and participants come in to the study. We're in fact following each individual over time clinically so that the cognitively normal people that have done this study, they don't all have exactly the same production in clearance rate. Some are faster and some are slower and so, the basic question is, are the people who have slightly impaired or impaired clearance but are cognitively normal now, are those people with increased risk of getting the disease at some point in the future?
Chris - How do you know that the people who have got Alzheimer's disease now haven't just got a reduced clearance because the disease has in some way damaged the brain, and the reduction of nerve cells that is associated with Alzheimer's disease has just impaired their ability to get rid of it? And that's why you're seeing that; As a consequence of the disease process, not so much as a consequence actually of having caused the disease.
Randy - With its current data, there's no way to distinguish between those two possibilities. And although we may hypothesise that for example, decreased clearance may lead to increased levels of amyloid beta and plaques, that's not demonstrated in this particular study. That's the point of doing the ongoing research, to determine if that's correct or not.
Ben - Randy Bateman, a neurologist who's looking for a way to knockout Alzheimer's disease on the head. He was talking to Chris Smith and has published that work this week in the journal Science.

14:25 - Glider Pilots Should Learn From Falcons
Glider Pilots Should Learn From Falcons
Gliding in all its forms has become a relatively popular pastime, and if you want to glide for anything more than a few minutes you have to take advantage of thermals, which are areas of warm air rising amongst the colder surrounding air. If you can keep your glider inside this column you will be lifted up without having to do any work.
The problem is of course that air is transparent so you can not see the thermals, and you are restricted to just feeling their effects. Therefore various rules have been worked out so that the glider stays in the thermal, essentially leaving you spiralling around, making the spiral larger if your rate of climb improves, so your wings are flatter, and making it tighter if it gets worse.
This system works well if you have a clean thermal, but if there is a lot of turbulence, you get temporary updrafts which is easy to confuse with a real thermal.
Zsuzsa Ákos and colleagues at Eötvös University in Budapest have been studying the flight of perguine falcons with attached GPS receivers. They have noticed that rather than always spiralling in one direction they occasionally swap direction, this sounds stupid as this will cause them to leave the thermal they are in, but in very turbulent air this means that they can test a larger volume of air for other better thermals, and if they find one they will stay in it, and in computer simulations it turns out to be better than the more conventional strategy.
This is of interest to glider pilots, but also to manufacturers of unmanned air vehicles which could save lots of energy by soaring, and nice simple rules like these allow them to soar without using up lots of energy calculating what to do next.
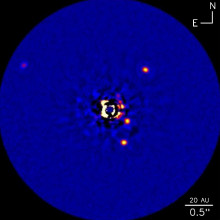
16:44 - Family of Four Planets Questions Formation Theory
Family of Four Planets Questions Formation Theory
Observations made by the Keck telescope in Hawaii have confirmed a fourth planet orbiting a nearby star but have thrown current theories of planetary formation into doubt.
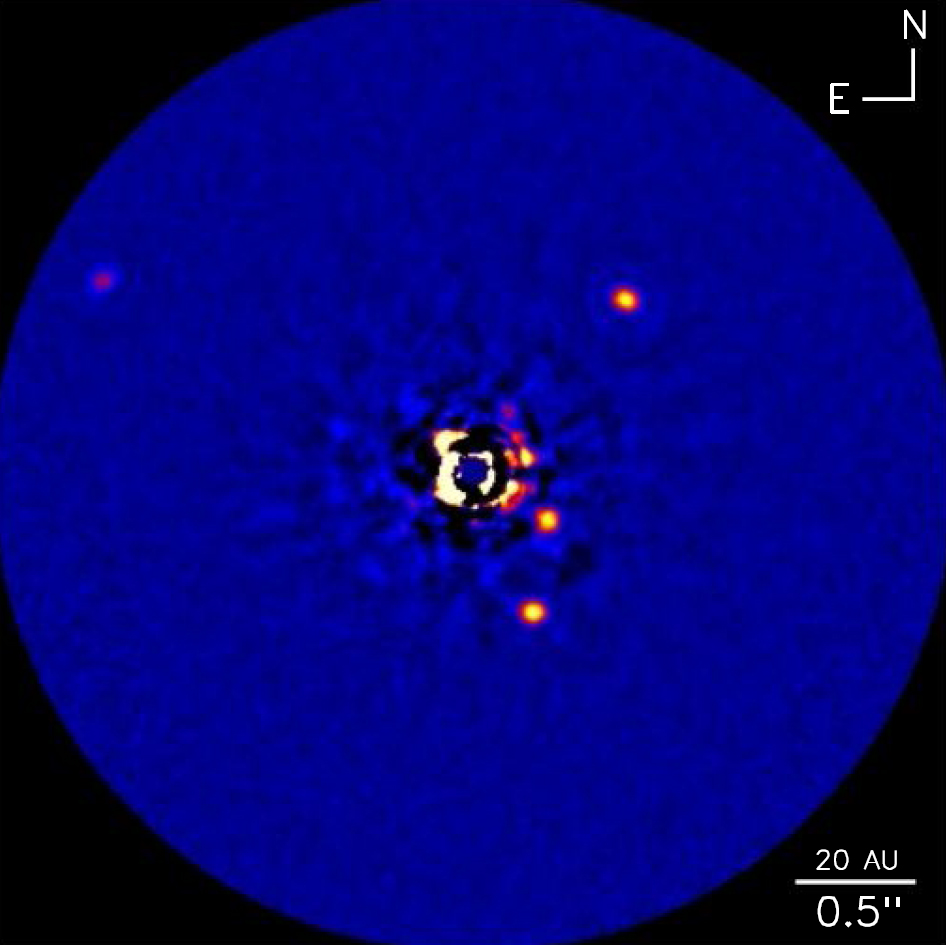 Publishing in the journal Nature this week Christian Marois, from the Herzberg Institute of Astrophysics in Canada, and colleagues in the United States analysed near infra-red images of the star HR 8799 and found a new exoplanet to add to the three previously discovered. These planets have particular properties that make this sort of imaging possible: They're very massive, the new one is somewhere between 7 and 10 times the mass of Jupiter; they're a long way from the star itself, over 25 times the distance between the Sun and the Earth; and they're relatively young, less than 100 million years. Younger planets are still hot from their creation, which makes them glow brightly in these frequencies.
Publishing in the journal Nature this week Christian Marois, from the Herzberg Institute of Astrophysics in Canada, and colleagues in the United States analysed near infra-red images of the star HR 8799 and found a new exoplanet to add to the three previously discovered. These planets have particular properties that make this sort of imaging possible: They're very massive, the new one is somewhere between 7 and 10 times the mass of Jupiter; they're a long way from the star itself, over 25 times the distance between the Sun and the Earth; and they're relatively young, less than 100 million years. Younger planets are still hot from their creation, which makes them glow brightly in these frequencies.
Finding new planets is always fascinating in itself but the really interesting thing about this system is that it doesn't fit with current models of planetary formation. There are 2 models that describe the formation of giant planets like this. There's the core accretion model, where small bits of rock collide and gradually clump together until the planet has enough mass and, therefore, gravity to hold on to an atmosphere. Then there's the disc instability or the gravitational collapse model, where variations in the disc of material around the star, the protoplanetary disc or proplyd, will condense out into balls of gas that gradually collect dusty matter at the core.
In the case of the HR 8799 system, the innermost planet could have formed by core-accretion, but those further out would need far longer; in fact, more than the age of the star itself. However, If we look to the disc instability model, the protoplanetary disc would not have been in the right condition to allow the innermost planet to form when it did.
So how did these planets get there? Right now, we can't be sure. The authors say that "a hybrid process with different planets forming through different mechanisms cannot be ruled out, but seems unlikely with the similar masses and dynamical properties of the four planets." The other option is that the planets formed elsewhere and then moved into their current locations, but until we find more systems like this one we simply don't have enough information to make a decision. But, with exoplanets being discovered at an astounding rate, we may find more of these planetary families soon.

19:20 - Sending Cheese into Space
Sending Cheese into Space
SpaceX a relatively small california based company has just launched a wheel of le brouére cheese into space, and more impressively brought it down after two orbits. Why... essentially just because they could.
The really impressive thing about it is that SpaceX which is a relatively small company mostly owned by Elon Musk who made a fortune out of Paypal and has used it to build space rockets. SpaceX has built both the Falcon 9 rocket and the Dragon space capsule themselves. This is the first time that a private company has lauched something into orbit and brought it back down again in one piece, and it is particularly interesting as the capsule is designed to be able to carry 7 crew at some time in the future. But for now spacex are concentrating on using the Dragon capsule to take cargo to and from the International Space station.
The cheese was sent up because they needed something to act as a payload, and there was no reason not to send cheese, I think it is rather refreshing and good for the space industry that someone is in a position to send cheese into space because they want to...

21:44 - Planet Earth Online - Monitoring Red Squirrels
Planet Earth Online - Monitoring Red Squirrels
with Tim Dale and Julian Chantrey, University of Liverpool
Ben - In this week's episode of Planet Earth we're looking at one of Britain's best loved mammals, the elusive red squirrel. They've been under threat from a squirrel pox for a number of years and in 2008, the virus decimated numbers of one of the few remaining reserves at Formby, near Liverpool. Sue Nelson visited the reserve to find out more about a four year scientific project, aimed at understanding squirrel immunity and improving red squirrel conservation. There, she met Julian Chantrey from the University of Liverpool School of Veterinary Sciences and Researcher Tim Dale, who was carrying a bunch of very long wire cages.
Tim - We've got four of the traps we use for the squirrels. They are in fact, mink traps but they generally use the same principle.
 Sue - So they're basically just a long rectangular wire cage, aren't they?
Sue - So they're basically just a long rectangular wire cage, aren't they?
Tim - That's right. With a pressure plate in the back that has a trigger that allows the door to spring shut when that pressure plate goes down. We usually bait the back of the trap with peanuts, maize and black sunflower seeds are quite favourable.
Sue - Where do these go?
Tim - We've got two tactics for catching the squirrels. One goes on the floor and the other one goes on the tree trunk vertically. They tend to work better for red squirrels whereas the ground traps work better for greys.
Sue - And once you've trapped the squirrels, what do you do then?
Tim - The first thing to do - because our first concern is the welfare of the squirrels - is just cover the trap. It tends to calm them down, they don't feel as frightened then and then we'll get all set up with this equipment and sample the squirrels.
Sue - Julian, once you've got a sample of blood from a red squirrel, what are you looking for?
Julian - We're looking for antibodies to the squirrel pox virus which shows that these squirrels have survived the epidemic back in 2008 and now have antibodies, a degree of immunity to this virus.
Sue - And so far Tim, what have you found?
Tim - We've found a small number of the population that do seem to have antibodies to the pox virus, which is certainly promising news because it does say that vaccine might be a viable option. In terms of numbers, it is a very, very small percentage and so, it isn't the answer to the red/grey squirrel disease problem.
Sue - When you say red/grey squirrel disease problem, is this because a grey squirrel's immune to this virus?
Tim - Effectively, yes. It seems that the grey squirrels brought the squirrel pox with them, when they came over from America. And because the grey squirrel has evolved with that virus for a long time, it doesn't cause any clinical disease. So, they're able to carry on a normal life without it affecting them.
Sue - It sounds as though this is potentially good news for the red squirrel population Julian, but I do sense, particularly from Tim, a touch of caution there.
Julian - Yes, you're perfectly right. You've got to be a bit cautious when you interpret these blood results. It's good that some of the squirrels have encountered the virus and survived, and now have antibody, but that doesn't necessarily mean that they are immune. All we can do is monitor the population as we are trying to do, making sure that their body conditions, their weight, their fat reserves are up to standard for them to survive through the forthcoming season. If another outbreak occurs, you'd hope that these squirrels will be the ones that survive and their offspring. But we just don't know without monitoring the group.
Sue - Tim Dale, you've got a brown cardboard box here at our feet. What's inside?
Tim - What we've got here is one of the red squirrels that we've chipped and sampled. When I say chip, that's an identichip so we can identify each individual squirrel that we've caught in the past and we're about to release it after its experience with us.
Sue - Okay. All right, are they quite shy creatures?
Tim - Definitely. We'll have to be reasonably quiet now...
Sue - Okay, you're opening the box. It's got lots of dried hay and straw in there, gently moving it. Oh my goodness! It is small, isn't it? There it went, up a tree. That gave me quite a fright there. It suddenly scarpers, doesn't it? Yes. Wow!
Tim - Yes, they don't hang around. They're fast little creatures.
Ben - Tim Dale and Julian Chantrey from the University of Liverpool, celebrating the release of a healthy red squirrel with Sue Nelson.
26:39 - Naked Engineering - Making Graphene
Naked Engineering - Making Graphene
with Andrea Ferrari and Antonio Lombardo, Cambridge University
Dave - We're talking about graphene this week, so to get a bit of background on what graphene is and how it was first discovered, I met up with Meera in an engineering lab at Cambridge University.
Meera - This week on Naked Engineering, Dave and I have come along to the CAPE building which is part of the engineering department here in Cambridge, and we're here to learn all about graphene. Now to start things off Dave, what really is graphene?
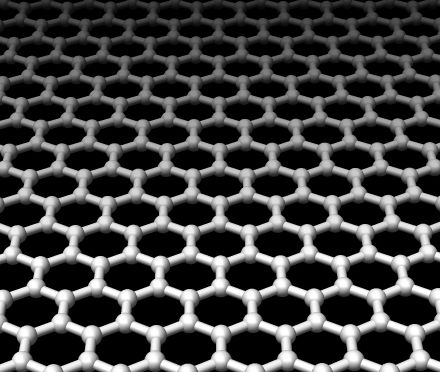 Dave - Well I'll start off with something much more every day, it's a pencil. Down the centre of a pencil, you've got what people call the pencil lead, but it isn't made out of lead. It's actually made out of graphite. This is a form of carbon and when you write with the pencil, it comes off and leaves a mark on the page. A part of the reason why it writes so well is that graphite is very, very slippery. This is because it's made of lots and lots of layers, all piled up, and the layers themselves are very strong, but the bonds between the layers are quite weak, so they can slip past one another and it's a really good lubricant. Now graphite has been around for hundreds of years, people have known about it, people made pencils out of it. It was only recently that people realised you can actually take one of these layers, a single atom thick, and separate it, and that's called graphene.
Dave - Well I'll start off with something much more every day, it's a pencil. Down the centre of a pencil, you've got what people call the pencil lead, but it isn't made out of lead. It's actually made out of graphite. This is a form of carbon and when you write with the pencil, it comes off and leaves a mark on the page. A part of the reason why it writes so well is that graphite is very, very slippery. This is because it's made of lots and lots of layers, all piled up, and the layers themselves are very strong, but the bonds between the layers are quite weak, so they can slip past one another and it's a really good lubricant. Now graphite has been around for hundreds of years, people have known about it, people made pencils out of it. It was only recently that people realised you can actually take one of these layers, a single atom thick, and separate it, and that's called graphene.
Meera - And well, one of the places where this graphene is being made is here in this clean room, and it's being made by Andrea Ferrari who's a reader in nanotechnology, and his team here in the engineering department.
Andrea - What is special about graphene is - there are two main things. The first one is it's a truly 2 dimensional material. We had nanotubes before, nanotubes were rolled up graphene sheets. It was quite unexpected to have a single layer and for it to be stable. The second thing that is very special about graphene is the combination of electrical and electronic properties. So the electrons of graphene behave as massless particles which means that we can have a huge mobility and extremely good electronic properties compared to any conventional semi-conductors and the second part is the optical properties that even being a single atom thick, a single layer of graphene can be seen by eye and absorb 2.2% of light.
Meera - An interesting aspect of graphene though is just how it's actually from graphite in the first place because it is just a single atomic layer...
Andrea - Yes. The first method we are going to see is the one that was originally developed at the University of Manchester by Andre Geim and Konstantin Novoselov and this simply consists of taking a piece of graphite, a piece of tape and peeling it off until you get a single layer, and Antonio is going to show you how to do it.
Meera - So with us is Antonio Lombardo, one of Andrea's PhD students...
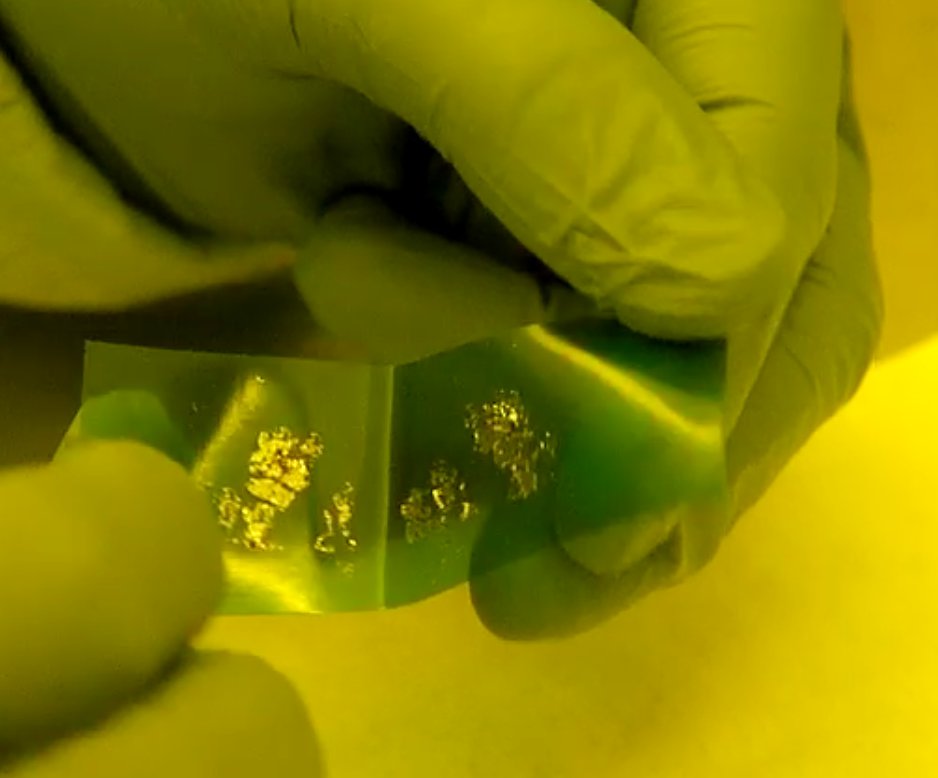 Antonio - Okay, I'm going to take one of these small pieces of graphite. I'm going to put them on top of some sticky tape. I'll use another piece of tape, just to clean, in a way, the surface of graphite many times until I get a nice and uniform distribution. So once I'm happy with the graphite on top of the tape, I just take one of these small silicon chips. These are covered in 300 nanometres of silicon oxide. Once we put the silicon substrate on top of the tape, there is some interaction between the top layer of the graphite and the silicon surface. At the end of the process, we have many different layers. This part is just one single atom thick.
Antonio - Okay, I'm going to take one of these small pieces of graphite. I'm going to put them on top of some sticky tape. I'll use another piece of tape, just to clean, in a way, the surface of graphite many times until I get a nice and uniform distribution. So once I'm happy with the graphite on top of the tape, I just take one of these small silicon chips. These are covered in 300 nanometres of silicon oxide. Once we put the silicon substrate on top of the tape, there is some interaction between the top layer of the graphite and the silicon surface. At the end of the process, we have many different layers. This part is just one single atom thick.
Dave - And it's just a remarkably simple process which - you could literally do this at home if you had a piece of tape and a lump of graphite!
Meera - So now, having found out how graphene could actually be isolated, we've now come out to find out ways that it can actually be used. So we've come to one of the optical laboratories, still here in the CAPE building. And so Andrea, what are some of the applications of graphene?
Andrea - Clearly, the first thing that people thought about was traditional electronics, replacing transistors and this may come in the near future. However, we are focusing here on applications that may come in the next few years, maybe three to five years, and one is for example, flexible electronics. So we can integrate graphene with plastic to make transparent and conductive materials that can substitute screens in laptops, and so on.
Dave - So because graphene is so thin, it's a very flexible material.
Andrea - Absolutely. Graphene is extremely flexible, it doesn't break. I guess if you take a piece of silicon and you flex it, you will break it. Graphene will never break. In fact, in plane, graphene is stronger than diamond. But still, it maintains the exceptional electronic and optical properties at the same time. So that's why it's an ideal material for flexible electronics.
Meera - Well you've got an example of one of your flexible electronics here in front of us. It looks like a piece of plastic really, about 8 centimetres squared.
Andrea - It is in fact a piece of plastic. Actually, two pieces of plastic and we put graphene on the top of it, and in between them, some liquid crystals. We use graphene because it's conductive, plastic is not. We apply voltage to the graphene and the graphene switches the liquid crystal on and off, and lets light through.
Dave - So the graphene on the two pieces of plastic is essentially attached to a battery of some kind. The two layers of graphene are applying quite a large voltage of the liquid crystal in the middle which is changing its optical behaviour entirely.
Andrea - And in fact, the plastic is just there as a container for the liquid crystal. It does nothing at all.
Meera - So at the moment then, it's a bit misty and it's placed over some writing on a piece of paper. You can't quite read the writing because of the misty screen.
Andrea - Now, we're going to apply the voltage and the writing will appear.
Meera - Yup! It's very clear.
Andrea - Yeah, it's very clear. It will become a completely clear window and in fact, utilising slightly different technology, we can also freeze it. So it's quite possible to freeze it on or freeze it off and that's what you would want in the screen of your office, or in a car, or something like that. On the other hand, if we actually put more contacts and put smaller squares, we can make a display, and we can display an image there, and change the image with time.
Meera - What makes this better than what's currently perhaps being developed or even in use?
Andrea - Currently, in all displays in televisions and in laptops, you use ITO which is Indium Tin Oxide or some similar material. These are becoming very expensive. In fact, most of the cost of a laptop is in the screen itself. And also, there's another very obvious implication that if you take your laptop and you bend it, it breaks. On the other hand, this one is made of plastic with graphene on the top. Graphene never breaks and so, it's flexible and works.
Meera - So how far away are we from actually seeing these screens in use?
Andrea - You see that here, with a team of just a few people, a collaboration between two groups, we can make already quite large bendable screens. In the same time, in Korea, Samsung has made larger screens based on a slightly different concept. And so, the prototypes are already here and I believe that in the next year or so, there will be already some prototype mobile phone based on this screen, and then the real question is, will this go to market? And unfortunately, that's something that I cannot answer. You need to ask a company about that.
Dave - That was Andrea Ferrari and Antonio Lombardo, explaining how graphene can be made using graphite and sticky tape, as well as demonstrating proof of principle that it could make really good flexible screens.
Ben - If you'd like to know a bit more about this you can find
a video of this sticky tape method in action.
Dave - And Naked Engineering is supported by an Ingenious grant from the Royal Academy of Engineering.

34:09 - When Sticky Tape is Not Enough - Ways to Manufacture Graphene
When Sticky Tape is Not Enough - Ways to Manufacture Graphene
with Dr Karl Coleman, Durham University
Ben - Using sticky tape is just one way of getting some graphene and it is not likely to be much use, as Andrea was saying, for Samsung making television screens. Exactly how graphene is made may well depend on what it is that you intend to use it for. So to explain more, we're joined by Dr. Karl Coleman. He's from the Department of Chemistry at Durham University. Karl, thank you ever so much for joining us.
Karl - Pleasure.
Ben - How do we make graphene in larger quantities? Sticky tape is all well and good for doing physics on graphene but how do we make it in bigger quantities, in bulk?
Karl - Yes. I mean, as you can imagine using sticky tape is a bit labour intensive if you wanted a large volume of the material. There are a number of ways to make it and we categorise those I guess into two camps. A top down approach, the sticky tape method would be a top down, so that's starting from graphite to the thin layers and then a bottom up approach. So top down, you can actually use a chemical method to really replace the using of the sticky tape. So there are people out there who basically take graphite and they do a rapid thermal expansion in the presence of an oxidising acid such as sulphuric acid. That breaks away, breaks apart those layers, and that also gives you the single sheets. So that's another way of producing it and of course, you can sort of imagine that being a little bit more scalable.
 Ben - So effectively, by superheating a block of graphite, rather than peeling layers off, you're causing the layers to break apart spontaneously.
Ben - So effectively, by superheating a block of graphite, rather than peeling layers off, you're causing the layers to break apart spontaneously.
Karl - Exactly. So, just give it a big kick in energy, through oxidising acid, split those layers apart and then you'll get some single sheets. It's a little bit more difficult to separate of course if you have lots of graphitic impurities in there as well. So there's lots of processing that's necessary, so lots of centrifugation, dispersing in solvents. There are other hurdles to overcome using that method. If you go for more of the bottom up approaches, which you can potentially see as more scalable, then we are talking more or so CVD, that's chemical vapour deposition approach. And there, you can actually decompose pretty much any hydrocarbon containing gas that you like; methane, ethane, propane, et cetera. People tend to prefer methane, but you can use a mixture of methane to hydrogen, and literally flow it over the top of a metal catalyst, and what the researchers in graphene like to do at the moment is use nickel or copper - copper being perhaps the most prominent. And you can grow a graphene film by the decomposition of the methane hydrogen on top of the copper, and I believe that's what Samsung are using, the Korean scientists, to grow very large tens of centimetre graphene films. So that's a little bit more scalable and easy to use than sticky tape.
Ben - And rather than using something that's becoming relatively rare as it gets used too much, the indium that we are seeing in current screens, using copper which is quite common and still relatively cheap, and something like methane or any hydrocarbon, of which the prices are slowly going up, but we do still have plenty.
 Karl - We do still have plenty. I mean, that's another issue about using fossil fuels, I guess. But yes, we do have plenty of natural gas kicking around. So using natural gas, you need hydrogen in there, you need a reducing atmosphere, and that's the key. And copper is perhaps the most useful because the solubility of carbon in copper is quite low. This is one of the reasons why you tend to grow only one or perhaps two layers on top of the copper substrate. It's not without its own difficulties, of course, because you'd have to remove the copper because the whole idea of replacing indium tin oxide, ITO of course, is to have a transparent electrode and copper isn't transparent, so you'd need to remove that copper and so it's usually an etch to remove the copper and then you can transfer the graphene film onto your plastic to assemble your plastic electronics.
Karl - We do still have plenty. I mean, that's another issue about using fossil fuels, I guess. But yes, we do have plenty of natural gas kicking around. So using natural gas, you need hydrogen in there, you need a reducing atmosphere, and that's the key. And copper is perhaps the most useful because the solubility of carbon in copper is quite low. This is one of the reasons why you tend to grow only one or perhaps two layers on top of the copper substrate. It's not without its own difficulties, of course, because you'd have to remove the copper because the whole idea of replacing indium tin oxide, ITO of course, is to have a transparent electrode and copper isn't transparent, so you'd need to remove that copper and so it's usually an etch to remove the copper and then you can transfer the graphene film onto your plastic to assemble your plastic electronics.
Ben - So, there are still fairly complicated methods that we have to create it. Is there one great way of doing it and several not so good ways or do they each have advantages over the other, depending on what product you actually want?
Karl - Yeah. It really depends on what you want to do and that will really dictate what method you want to use. The micromechanical methods, i.e. the scotch tape, sticky tape is very good for physicists, they need a small little chunk of the graphene, or a small sheet and they can study it, measure it, and do lots of different things. The display is obviously a little bit different so you are going to need something like a large piece of copper foil to grow your film on, but then you have to transfer it from that copper onto your plastic and remove the copper. So yeah, I mean if you want the ITO replacement, that would be the method you would use. But if you were to use it in say, composite materials, there's a lot of work going on at the moment about using graphene in composites so as a nanofiller. Then of course, neither of those methods would be suitable and so we need another method to make the graphene. There are variations on CVD to do that, so where you don't actually use a copper foil, you use other metal catalysts or you use a spray pyrolysis type approach to get basically graphene powder that you would then be able to disperse in your polymer matrix to make your composite material. So it does really depend on what you want to do with it.
 Ben - Composites are relatively new, we haven't been using them for that long, but they've made huge waves in the aerospace industry. We've had a question from David Whalley who got in touch via Twitter. He wants to know if graphene is likely to be used in spacecrafts. I would imagine it's composites we'd be looking at there.
Ben - Composites are relatively new, we haven't been using them for that long, but they've made huge waves in the aerospace industry. We've had a question from David Whalley who got in touch via Twitter. He wants to know if graphene is likely to be used in spacecrafts. I would imagine it's composites we'd be looking at there.
Karl - There definitely would be composites. On its own, it would be difficult to use in aerospace. So, it would be incorporated into some sort of polymer matrix. So yes, there's lots of carbon based nanofillers. So of course, we are all familiar with carbon fibre which is perhaps the most common one that's out there at the moment, and carbon nanotubes which have been making some headway, but of course, there are some problems associated with that. I think really, people have been looking there at these one dimensional, high aspect ratio materials as fillers. And then graphene has come along and challenged carbon nanotubes in the prospect of being a carbon fibre replacement. So, you'd have to get it into some sort of polymer matrix, so a resin or a thermo plastic, and really, the difference I guess with the nanofillers is you require much less of the nanofillers compared to say, carbon fibre. And so, that opens up a whole new game for the guys dealing in composites, and how they process the materials, how they mould complex shapes, et cetera. So it opens up new doors perhaps that you wouldn't get with carbon fibre, but you'd still get the high strength:weight, at a ratio that you would need from a composite material.
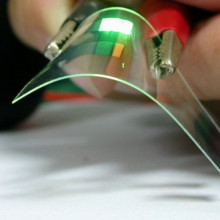
41:36 - Applications of Graphene
Applications of Graphene
with James Tour, RICE University
Dave - Graphene seems to be a very versatile material that we should soon see turning up in a range of applications. Now we're joined by James Tour who is Professor of Chemistry, Mechanical Engineering, Material Science, and Computer Science at RICE University which is an incredibly impressive list. James, thanks for joining us. So, what is it about graphene which makes it so useful? What are its properties?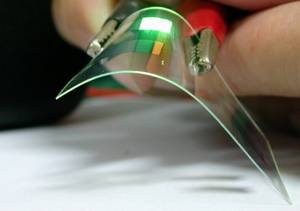
James - Well, it is certainly projected to do many things. I think certainly from an electronics perspective, it can carry an enormous amount of current, far more than copper or gold for similar sized structures. It has a very high mobility which means that you could build very fast switching devices, and that's really important if you want to be able to do high performance computing. The numbers are just frighteningly high. If you consider a mobility of about 500 per silicon, graphene has been shown to be up around 1 million. So, it could operate with much faster switches, and then also on the display side, but then also working in conjunction with fluids.
Dave - So you were talking about mobility then. What actually is mobility?
James - So, I guess a way you can imagine this is if you switch a device and you move the carriers all in one direction so they're propagating across, you have to wait for those carriers to get across to get your message across the silicon. So you can't switch it back the other way until the carriers have gotten all the way across in one direction. So there's a time delay. You can't switch it as fast as you'd like. You have to wait for things to get across it. But if things can get across extremely rapidly, then you could keep switching far more rapidly and you could build much faster switches.
Dave - So, a major limit on the speed of a computer chip at the moment is just the time it takes for the signal to get from one side of the transistor to the other side of the transistor and graphene you say was more than a thousand times faster than silicon. So, how difficult is it going to be to build a computer chip out of graphene?
James - Well, you can certainly build one transistor. Lots of people are doing that, but certainly in our computers, we have billions and billions of these, tens of billions, hundreds of billions of them. So, nobody has solved this one yet, but the nice thing about graphene which you could not get with carbon nanotubes is that graphene, if you make a sheet of it, you can chop it up using resists and light, much like you do with silicon, so you can pattern in that way. So you make a large area and you chop it up into the smaller pieces that you want, and that's something you could never do with carbon nanotubes. The problem still remains however in that it is a planar structure and generally, what you want to be able to do is build vertically. So it's analogous to having straws. If your straws were laid out on a surface, you could only get so many, but if the straws are standing up, you could pack a lot more and right now, silicon is assembled vertically and not horizontally so that you can get more on a chip whereas graphene is predominantly horizontal, and that's the way it's going to be until we get better at growth methods and transfer methods that might allow it to be vertical. So, there's still many hurdles that need to be done before this is going to begin to rival silicon.
Dave - So at the moment with graphene, even something as simple as making one wire across another would be very difficult.
James - No. You can make one wire across another. Graphene would be good for that, but in a vertical array like silicon, then it's hard. But that's taking on silicon at the high end electronics and that's not really fair to graphene because silicon has had 50 years of work on it and that is probably millions and millions of person years put into it, and many, many trillions of dollars or pounds put in to it. So, there's a lot of development that's got into silicon. Graphene has a fundamental property that's really quite amazing and in lower level applications, it's going to compete quite well, such as in the touch screen displays that we've heard about, being able to roll these things up and fold them up which would be quite nice, so that when you drop your iPhone, you don't have a cracked screen which is a real headache for the user.
Dave - Because up until now, the standard transparent electrode is indium tin oxide, it is sort of ceramic, isn't it? It's very brittle whereas I guess graphene is so thin that it's got to be flexible.
James - Yes, it's flexible and so that you can put it on a flexible substrate, so that you can think of rolling up your iPhone and putting it behind your ear like a pencil or whatever, and walking away. I mean, that's certainly the possibility.
Dave - So you can actually make all of electronics with graphene. I mean, graphite behaves like a metal. It just conducts electricity, so can you turn graphite into a semi-conductor as well as conductor?
James - That's a good point. Graphene is a metal and it has a band gap that's zero or near zero so, you have to turn it into a semi-conductor. That can be done by putting in impurity atoms into it and then you can begin to open up a gap. We don't have the absolute control over that quite as we would like, but researchers are getting better at that all the time. So, I think that that's going to open up, but again, I don't think that that's going to be the forefront of applications and I think that applications are going to insert in an even easier market than mainstream electronics.
Dave - So, this is going to build the transparent stuff. In fact, Barry Freeman was saying, "Is graphene going to be commercially viable any time soon and when will we likely see it be used?"
James - Well again, I think some of the simpler applications are for example, one of the things that we're working on were taking both graphene and the oxidised version of it, graphene oxide, and putting it into drilling fluids, fluids that are used to drill holes in the ground where they find oil. And one of the problems is the fluids infiltrate the rock and then when you try to yield the oil from the rock, the rocks have often been plugged and they have to go back in and try to wash these fluids out. We found that if we could add the graphenes in oil based drilling fluids or the graphene oxides in water based drilling fluids, these act as little nanofilters and keep the drilling fluid from infiltrating the rock. This is a very simple application, so any time you're talking about nanotechnology, you have high level of applications and then you have simpler applications. So you have both active applications where you're asking a lot of the material, asking it to have mobility, asking it to accept an electron and spit out a photon for example, or you have passive applications where just its mere presence, acts as a filter or something. It's a very passive application so it can insert far more easily into the industry, and I think that that's where we begin to see the initial applications. So again, a composite is a passive application, its presence being there, causes the composite structure, the molecules and the composite generally to be stiffer because they have to align past the nanostructure, and that increases the performance of the composite in that way. So the simpler applications will always insert first.
Dave - Brilliant! Thank you very much, James. That was Professor James Tour from the RICE University.
Can Graphene be converted to Diamond-phene?
Dave - I'll start off with diamond and graphite, the large scale structures - three dimensional structures. Graphite and diamond have got incredibly different structures even though they're both just made out of carbon atoms. In graphite, you've got a whole series of planes made up of hexagonally arranged carbon atoms, all tesselating together with an atom where all the hexagons meet. Whereas diamond is this tetrahedral structure with each carbon atom being bonded very strongly to four others at the corners of a tetrahedral pyramid or triangular based pyramid. This is the reason why diamond is incredibly strong. It bonds in every possible direction whereas graphite is very strongly bonded inside the plains, but very weakly between them, so they slide across each other, and it's quite a good lubricant.
Ben - So, if you wanted to convert between the two, you actually have to break and then remake quite a lot of bonds, so it must be quite an intensive process?
Dave - Yes, that's right and diamond is only actually stable under very, very high pressures. The stable form of carbon at room temperature and pressure is graphite. So, in order to convert graphene or graphite into diamond, for a start, you'd have to use incredibly high pressures. And also, diamond is an intrinsically three dimensional structure so a single plain of it, equivalent of graphene, wouldn't really make sense. It will be incredibly unstable and it would immediately convert back into something like graphene or just fall apart.
Ben - So, graphene itself is not a better source for making something like a flat sheet of diamond. You may as well just use any old carbon kicking about.
Dave - Yeah, that's right and it's probably a lot easier to get it from some kind of gas like methane or something in a CVD process like they've talked about earlier.
Why do we Consider Graphene to be Two Dimensional?
We posed this question to Dr. Karl Coleman from Durham University...
Karl - I guess you could argue it has a third dimension, but, one atom thick is really the thinnest you can go. So, I would argue that it is negligible in thickness in one direction and that is why we call it a 2-dimensional material and graphite of course would be the 3-dimensional version where you have several layers that make up the structure. So basically, you can't get thinner than one atom I would argue.
Dave - And I guess also, the electrons actually have a wavelength, so in effect something a bit like a size and the wavelength is of the order of the thickness of the graphene. So, to the electron, it does look like a 2-dimensional thing because it is as thick as they are.
Karl - It is. It is confined in that 2-dimension. You're right, yes.
How does Graphene compare to Buckminsterfullerine?
James - So, if you have a bucky ball, that is considered zero dimensional. Even though it does have dimension - it doesn't reach out in different directions. If you have a nanotube, that's considered one-dimensional. It is extending in one direction and in the other direction, it's only about a nanometre wide. But fundamentally, it's considered one-dimensional whereas graphene is like a sheet, it's 2-dimensional. The fullerenes turned out in high compressive operations, they would actually tend to decompose in many structures and that's why they turned out not to be very good lubricants. Whether graphene is going to be a great lubricant, I don't know. Graphite, a 3-dimensional structure, is a good lubricant because it's able to shear off lots and lots of sheets of graphene which is really what makes it slippery.
Ben - So, the sheets themselves slide past each other, which is also why they make good pencil lead.
James - Correct, and whether a sheet of graphene would make a good lubricant, I'd rather doubt it in the sense that it does not have that ability to slide past itself. It is only one atom thick.
53:50 - Is Blood Magnetic?
Is Blood Magnetic?
We spoke to Stuart Richmond from the Department of Health Sciences at the University of York to answer this question for us...
Stuart - The fact that blood contains iron is one of the reasons why some people believe magnetic bracelets might have an effect on the human body. However, blood is not magnetic in a conventional sense. In other words, it is not ferromagnetic which is what most people understand as magnetism.
If blood was ferromagnetic, then people would bleed to death or explode in MRI scanners which produce much stronger magnetic forces than those of magnetic bracelets.
So, although deoxygenated haemoglobin is paramagnetic and very slightly attracted to a magnet, and also both oxygenated haemoglobin and plasma are diamagnetic or in other words, slightly repelled by a magnet, in theory, wearing a magnetic bracelet shouldn't have a physiological effect.
Firstly, any influence in the polarity of ions within red blood cells would be lost because blood flows in a pressurised and turbulent way.
Secondly, blood is warm, so for any paramagnetic effect to occur it would need to overcome the forces of brownian motion. All of which are extremely unlikely.
So, we turn to the second part of the question, do magnetic bracelets actually work? In my research on magnet therapy in arthritis, I began not by asking how magnetic bracelets might work, but rather by testing whether they had any health effects on humans and by trying to control for the power of imagination. The best available evidence showed that magnet therapy lacks any meaningful effect other than a placebo effect for arthritis and pain control. Although there are some contradictory results, it would appear that for those trials which have shown a benefit that have also tended to suffer from problems of blinding which might explain those findings.
Diana - And when he says that test subjects weren't blind, that means that they were able to identify if their bracelet was magnetic or not, potentially altering the outcome of the trial.
Stuart - So, despite this, the effects of positive suggestion should not be discounted if people choose to believe that wearing a magnet might help, then it may well do. Although there are no known side effects, the danger is however that people may use magnetic bracelets instead of other clinically effective treatments.
- Previous Sending Cheese into Space
- Next Making Graphene









Comments
Add a comment