Alois Alzheimer, who described the first case of the disease now named after him, would have been 150 years old this week. But what have we discovered about the disease since he presented the first Alzheimer's case over 100 years ago? And how can fruit flies, arm hair and video games untangle the most significant threat to our generation? Plus, in the news, how making mosquitoes male could reduce malaria, protecting astronauts from solar radiation, and why is beetle sex a sticky situation...
In this episode

00:54 - Modified mosquitoes that only make males
Modified mosquitoes that only make males
with Nikolai Windbichler, Imperial College London
For years people have been trying to wipe out malaria by using insecticides to  control mosquito populations, but they can develop resistance to the chemicals, and the disease still kills hundreds of thousands of people around the world every year.
control mosquito populations, but they can develop resistance to the chemicals, and the disease still kills hundreds of thousands of people around the world every year.
Now scientists at Imperial College London have genetically engineered mosquitoes so that they only produce male offspring; and that's important because it's the females that bite and spread disease.
Kat Arney went to see Nikolai Windbichler, who led the research, to find out how changing the ratio of male to female.
Nikolai - Well the idea is, if you progressively shift the sex ratio of a population towards males and there are fewer and fewer females in the population then the overall size of the population will decrease up to a point where the population cannot sustain itself anymore and will actually crash.
Kat - What did you to try and skew this sex ratio?
Nikolai - We introduced into the mosquito a gene which essentially destroys the X chromosome. As you know, as in humans, so also in mosquitoes, there are two types of sperm produced by males, sperm that carried a Y chromosome and sperm that carried the X chromosome. The sperms that carry the Y chromosome produce sons. The sperms that carry the X chromosome produce daughters. So, we found a way to specifically eliminate the sperm that carried the X chromosome so that only the sperm that carried a Y chromosome would be functional and this male would produce only sons.
Kat - This sounds really clever. How did you do that to target and destroy the X chromosomes?
Nikolai - What we found is essentially an Achilles heel, a genetic Achilles heel of the malaria mosquito. There's a certain DNA sequence that is present in many, many copies on the X chromosome only and nowhere else in the mosquito genome. So, we use a DNA cutting enzyme that cuts the chromosome. You can imagine like with a scissor, and in many, many places, it cuts the X chromosome. So, this chromosome becomes destroyed. It's no longer functional.
Kat - So, you've these kind of lines of mosquitoes that have these DNA scissors in. What happens?
Nikolai - And then what happens is, as you can imagine an enzyme that cuts DNA, targeting the X chromosome is lethal for most cells. But we are making sure that this chain is active only when sperm are produced. So then what happens is the sperm that would have carried the X chromosome in those sperm, the X chromosome is destroyed. But the sperm that carried the Y chromosome, they do not have to sequence that we're targeting. So they're totally unaffected. So, they are produced normally and transmitted to the female and you have male offspring.
Kat - The next generation is mostly all male offspring. They're going to be looking for some ladies. What happens then?
Nikolai - Well, one thing that we probably should add is that this gene is also transmitted to the offspring, but only to half of the offspring. So, half of those males looking for ladies, they will do the same thing again in this generation. They will produce only sons. Over time, progressively over many generations, there are fewer and fewer females in the population. And eventually, there are no females in a population and the population crashes.
Kat - So, this could be a really fantastic way of basically getting rid of mosquitoes. They're the bane of my life. They always bite me. I hate them. What would be the consequences of completely getting rid of a population of mosquitoes, say, in a town or even a country?
Nikolai - There are many. There are 3,500 different species of mosquitoes. We are only targeting one specific species which transmits malaria to humans. So that's in a sense a much more targeted intervention than for example insecticides is the current control method which targets all sorts of insect species.
Kat - Obviously, this could be fantastic if you could get rid of the mosquito populations and that would eradicate malaria, but how close is this to being something you can roll out and are there risks of releasing these modified mosquitoes into the wild?
Nikolai - This is a very promising technology, but we're still many steps away to rolling this out. We have both technical hurdles to overcome still, but as I have to make sure that all aspects of biosafety, ethical concerns and regulatory concerns would be addressed before we go any further with this. The next step is to take these mosquitoes and test them at a large scale. We have tested this technology in small population cages, but we have a facility in Italy where we have larger, cages to the more field-like controlled environment and there, we want to test the technology to see how it performs.
Kat - What's it like working with so many mosquitoes around you? Does it make you a bit twitchy?
Nikolai - Yeah, some people get quite twitchy.
Kat - How much do you get bitten?
Nikolai - We get bitten once in a while but it's really alright. It would be the same if you go in a camping trip probably.
05:54 - Radiation shields in space
Radiation shields in space
with Ruth Bamford, Rutherford Appleton Laboratory
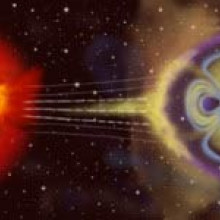
Looking heavenwards now, one of the biggest dangers while travelling in space is 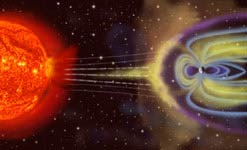 exposure to potentially lethal bursts of radiation from the sun. These can flare up during periods of increased solar activity known as solar storms.
exposure to potentially lethal bursts of radiation from the sun. These can flare up during periods of increased solar activity known as solar storms.
Currently, we have no way of protecting astronauts. The Apollo moon missions in the 60s and 70s avoided them purely by chance.
But now, the moon may have provided us with an answer: dark patches on the surface - known as lunar swirls' - have given physicists an idea for a way to shield our spaceships in the future. Ruth Bamford is heading this work at Oxford's Rutherford Appleton Laboratory, and explains why this radiation is such a problem...
Ruth - Well, this particular type of radiation consists of energetic particles that are more akin to what you would see in a particle accelerator. And they are so fast they can go through the hole of a spaceship and smash the DNA of the astronauts. During a storm, so many of these can occur that it's beyond the body's capabilities to repair.
Chris - When you say there can be so many of them, what's the likelihood of someone on a space journey coming into contact with these sorts of levels of radiation?
Ruth - The levels occur during a storm and those storms are intermittent in their occurrence. So on average, the sun will give out two or three of these big events a day during solar maximum and about the same during solar minimum a week. They don't all come our way, but if you are in the way and you're on a long journey for a year and a half to Mars then the chances of one passing through the spacecraft are too high to be acceptable for the astronaut's safety.
Chris - Why can we stop this radiation because we can do that on Earth?
Ruth - Well, we have the Earth's magnetic field producing a barrier, a first line of defence and then we have the Earth's atmosphere. We can't put large quantities of material on a spacecraft because it would be too heavy. So, things like lead or concrete which is what you might consider make a good shelter on the Earth won't work in space.
Chris - We obviously can't cart those up into space because they're far too heavy. So, what could we do instead?
Ruth - Well, we have to get smarter and this is where the examples of what nature has offered us comes into play. We start with a magnetic field, but if you just use a magnetic field to deflect charged particles, because they're so fast, you'd need an enormous magnetic field to do the job. So, what we do in fact is, we're proposing you gather the substance called plasma from the solar wind and use it, captured by the magnetic field to help hold back the charged particles by adding an electric field to your magnetic f ield.
Chris - Okay, well let's unpick this a little bit. So, you've your spacecraft going around in space. Talk us through the first thing you'd have to create to create this shield you have in mind? What's the first thing you got to do?
Ruth - Like the Earth, we start off with the magnetic field. So, the Earth has a magnetic field. It's very low intensity magnetic field even here on the surface of the Earth, but it's still capable of holding back this radiation from space. The way it does it and the way we're proposing to be able to put one on the spacecraft is by gathering the solar wind particles which are in the vacuum of space. So, between here and Mars, it isn't really a vacuum. In that sense, there's a sparse number of protons and electrons, the component parts of hydrogen atoms are coming naturally from the sun, that are flying off in the solar wind. And we're suggesting that you capture these and hold and control them by the magnetic field that you bring on-board with you.
Chris - So, you have a spacecraft which has its own magnetic field. It's generating a bit like the Earth's magnetic field, this will capture some of these particles that are natively floating around in space, these protons and electrons, and once you've got them stuck into this magnetic aura around your spacecraft, then what do you do?
Ruth - Then you've got an electric field helping the magnetic field and you'll produce a narrow electric field some distance from the spacecraft. So, the spacecraft is safe from this electric field. The idea is that, much like you would deflect a charging rugby player with an ankle tap or push to touch, what you need is enough of an electric field that will deflect rather than stop these hazardous energetic particles that would otherwise go through the spacecraft.
Chris - I see. So, the capture of the charge particles in space gives you that electric field which then extends the reach of the protection you've got around your spacecraft so any more stuff that comes flying past gets enough of a push so it's off course and it misses or swerves around your spaceship. It doesn't go through your astronauts inside.
Ruth - Yes, that's the idea. During a really big storm, you can release some of this plasma yourself from the spacecraft, from a gas canister and even for a mission to Mars, the quantity of gas you would need, being no bigger than say, a fire extinguisher. So you wouldn't need vast quantities.
Chris - How would you power the magnetic field in the first place? Would that be solar or a nuclear or something to give some source of magnetism to start the process off in the first place?
Ruth - You would need a magnetic field on-board the spacecraft and the suggestions are that the superconducting magnet would be the most practical. It would be powered by whatever is powering a manned spacecraft which currently, they suggest it wouldn't have to be a nuclear power source because with a manned mission, you're going to require quite a lot of power in general.
Chris - This is theoretical at this stage, isn't it? Have you got any plans to - or anywhere you can test this?
Ruth - Well, we have tested it in small scale in the laboratory and we've also been looking at natural mini-magnetospheres - as they're called - on the moon. This has shown that it does actually work in space and at the dimensions that we needed to. There's a little feature on the moon, on the eye of the man in the moon, his left eye, a bit like a tear. It looks like a patch of white on the dark area of the moon, and that little patch of white is called Reiner gamma. It is being protected by a small patch of magnetic field that naturally occurs on the moon because the moon doesn't have an overall magnetic field of its own. It would appear that for the eons that it is being there, that little magnetic anomaly. It's been able to keep away all these hazardous particles sufficiently to change the colour of the moon.

12:32 - The Turing Test
The Turing Test
This week a computer program reportedly passed the 'Turing test' for the first time, tricking people into believing it is human. This was part of a competition run by Reading University to commemorate the 60th anniversary of death of the test's creator: Alan Turing. Here is your Quick Fire Science on the Turing test...
- In 1950, Alan Turing developed a way to test a machine's ability to mimic human behaviour. He called this 'The Turing Test.'
- In the test, a human judge writes to a subject in another room. This subject can be a person or a machine.
- The aim of the test is for the judge to suss out whether the subject is human or machine, whilst the computer tries to dupe the judge into thinking it's a real person.
- When creating this test, Turing made a prediction. He thought by the year 2000, machines would be able to fool 30% of human judges in a five minute test.
- However by 1966, a programme called ELIZA appeared to pass the test. It used keywords from the judge's typed comments and transformed them into new sentences by following a series of rules.
- Versions of ELIZA, now known as chatterbots, are found on the internet today and they continue to trick people into believing they are human in the hope of gaining sensitive personal data.
- The latest computer program to have met Turing's predictions is called Eugene Goostman.
- The Russian-developed software emulated a thirteen year old Ukrainian boy who didn't speak much English and spoke to judges, via webchat, for 5 minutes.
- Eugene managed to fool 10 out of 30 human judges, fulfilling Turing's prediction.
- Turing never actually specified the percentage of judges that needed to be fooled for a computer to pass so it is debatable whether programs can ever be said to have passed the test.
- Some have argued that Turing's test was proposed as more of a philosophical question about a computer's abilities to mimic humans than an accurate test for artificial intelligence.
- If you want to try your luck against Eugene you can visit www.princetonai.com/bot

15:05 - Beetle sex is a sticky situation
Beetle sex is a sticky situation
with Victoria Gill, Science Journalist
Sex is a tricky business for some organisms, and particularly the Great Diving  Beetle, which has to cling onto its mate underwater. But, in solving this slippery problem, mother nature might have inadvertently come up with a recipe for an underwater sticky tape, as Victoria Gill explains...
Beetle, which has to cling onto its mate underwater. But, in solving this slippery problem, mother nature might have inadvertently come up with a recipe for an underwater sticky tape, as Victoria Gill explains...
Victoria - This is a study that comes from the Royal Society's Journal Interface and in this case, they're looking at beetle feet. Now, these are particularly amazing beetles because they're diving beetles. These quite large carnivorous beetles, though they breath air at the surface. They spend most of their life in the water. That means that they have to mate underwater as well. How they do this is when they're in the water, they mount onto the females and they have to keep hold of the females in this aquatic environment and they've actually evolved special limbs that stick to the female's bodies to do this. So, that's what this team were asking. They wanted to work out how these feet stick to the female beetle's body in this reversible way because if the male stays stuck to the female, he might not be able to get to the surface to breathe and they figured out exactly how this amazing beetle feet work.
Chris - How do they work? What do they do?
Victoria - So, they've actually looked at a very, very fine scale with microscopes, essentially filming these beetle feet attaching and detaching from the female body, and they've measured the forces that are produced, the strength of this attachment. They produce these amazing videos that you can see on the Naked Scientists' website. What they reveal is that there are physical structures on the bristles on the ends of the beetle's feet - tiny little plunges or suction cups. So, they looked at two species and one of them - the more primitive species - has little spatulas on the ends of these bristles that sort of act like a reversible tape that sort of slide over the female's body and seem to have some kind of adhesion and they slide around and then peel off. The other more evolved beetle has even more amazing bristles on its feet. It has tiny little - what look like sink plunges - the little circular suction cups that actually stick to the female with this pressure and then can be just pulled off and released. They look just like a sink plunger. It's really remarkable at the fine scale.
Chris - If they stick on and they stick on really hard, how do they get them off again?
Victoria - That was one of the things that surprised them because the more primitive beetle has what just looks like a bristle with a flat end. It looks kind of like just a spatula that sort of slaps down onto the female's body. But actually, it has these little fine hairs that attach like sticky tape and it has channels on these hairs that allow liquid to seep through. So, it's like an adhesive sticky tape that seems to work underwater. So, that's how that works by peeling those spatulas slowly off the female's body. They can detach easily. The suction cups, it's literally like a suction cup device that we might stick up a sign onto a window or something like that. They just attach and then they pull off. So, you can see it in these videos. They put one of these suction cups onto a female's body and then they just pull it off and you just see that with additional force, they have to sort of release themselves with a bit of a tug but it just pops off.
Chris - This is all very interesting from a biology point of view, but I can hear the man and woman in the street saying, "Why are we spending..." Well Chinese taxpayer's money looking at this, but why is this useful?
Victoria - Well actually, biomimetic in general is an area that's really, really useful in terms of robot design. There's a lot of designers of flying robots and drones that are looking at the way animals fly in order to produce more efficient and smaller and more miniaturised drones. Spider silk is another classic example where nature can do something that we can't do, really produce something that's incredibly stretchy, incredibly tough, and incredibly strong. This is another challenge. Actually, sticking things together underwater is really, really tricky. We've got loads of adhesives and engineering devices that can stick things together in air, but aquatic environments make things like adhesives really, really difficult. So, if we can see how animals are managing this in a reversible, really efficient way as part of their life and part of their method of survival, then this is very fundamental start to being able to do something that we'd be able to use in engineering. You can imagine underwater engineering in terms of shipping, in terms of bridge building. It's actually really important.
19:17 - 3D print shows king had scoliosis
3D print shows king had scoliosis
with Piers Mitchell, Division of Biological Anthropology, University of Cambridge
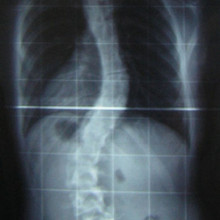
Scientists at Cambridge University have used a 3D printer to reconstruct the spine 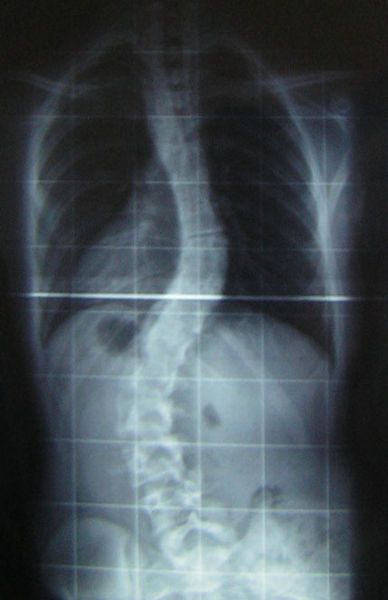 of King Richard III, whose 500 year old remains were discovered buried beneath a car-park in Leicester in 2012. The results, which were published in the Lancet medical journal, confirm that the king had an s-shaped twist to his spine called scoliosis. Graihagh Jackson asked Piers Mitchell, from the University of Cambridge about what was found.
of King Richard III, whose 500 year old remains were discovered buried beneath a car-park in Leicester in 2012. The results, which were published in the Lancet medical journal, confirm that the king had an s-shaped twist to his spine called scoliosis. Graihagh Jackson asked Piers Mitchell, from the University of Cambridge about what was found.
Piers - The body itself was excavated by Joe Appleby who works at the University of Leicester. She was as surprised as everyone else to find this skeleton so early on in the excavation of the friary. She identified that there was a burial lying on its back that had a clear spinal deformity. What was interesting was that the grave wasn't quite long enough for him so that his head is all scrunched up. So, it looks very much like this was a fairly hastily buried person and not someone who was buried in pomp and circumstance as we might expect a ruler who died when there wasn't going to be a change of dynasty.
Graihagh - So, describe to me what we're seeing in this picture and what led you to believe that this might be in fact King Richard.
Piers - When you look at this burial, we can see that there's a marked curve to the spine in the thoracic area of the spine. So, that's the part of the spine where the rib cage is and this shows that this person had a deformity of their spine in life.
Graihagh - So, the spine was a particular interest. What sort of process did this spine undergo?
Piers - The first thing to do was to look at the spine in the ground, take appropriate photographs so we can then measure angles later. And then having excavated the skeleton and cleaned all the bones, we can look at each individual bone and see if they look normal or abnormal. Then the bones underwent further imaging with CT scanning which allows you to do a 3D reconstruction virtually on a computer. The guys at Loughborough used a clever programme to then print these bones using a special plastic polymer. So, the printout basically looks like white plastic bones. And then to recreate everything, my colleague at Leicester, Bruno Morgan, took the bones and re-aligned them and this then allowed us to create a 3D version of the spine. Because when you look down at a burial, you can only really see it in 2 dimensions.
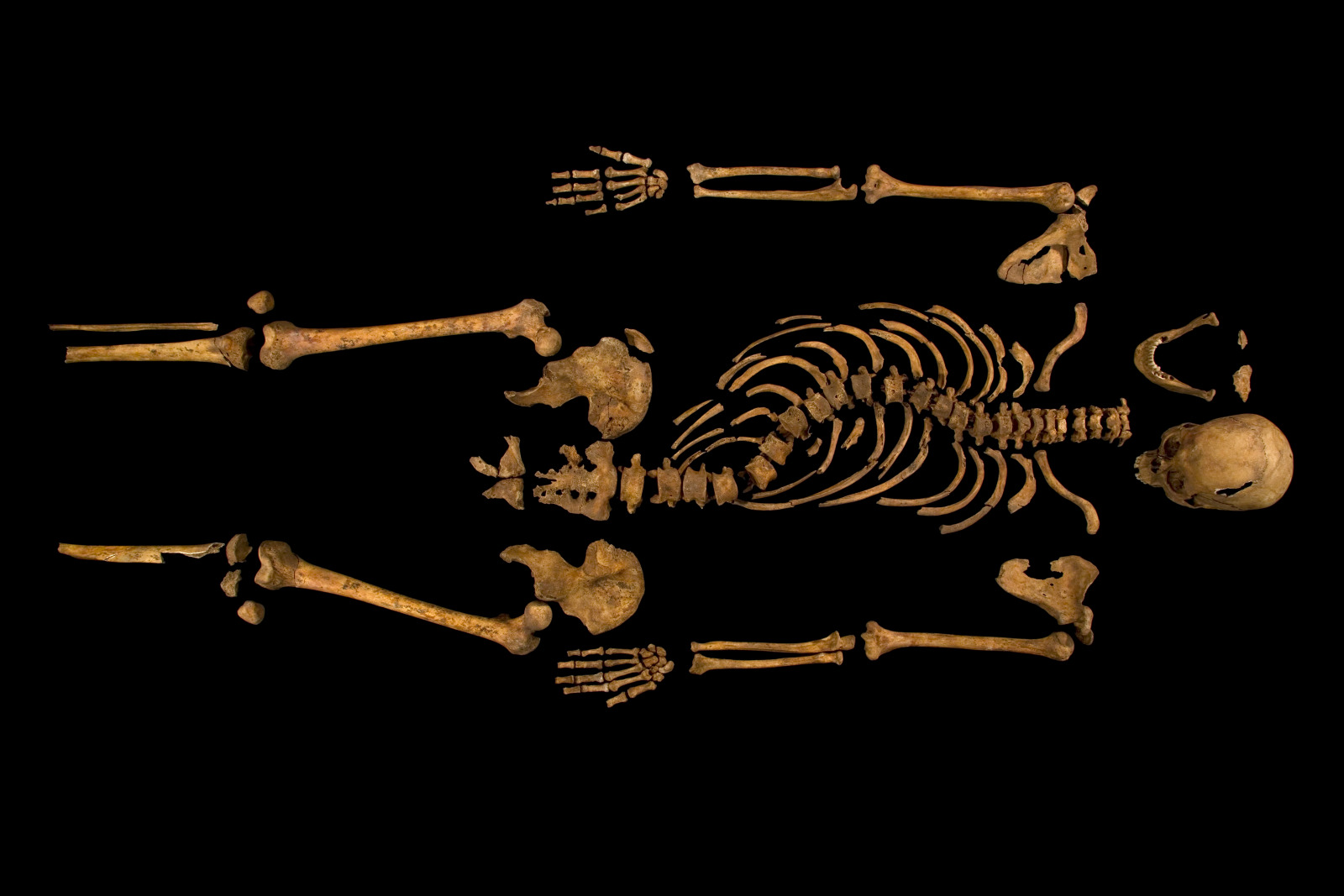 Once we created a 3D recreation of the spine, we can actually see that this was a spiral twist. That's very much what we see in modern patients today with scoliosis. The original photographs showed that he had a scoliosis which had about 70 to 80 degrees of curve which is quite significant. Using modern evidence from patients who have a scoliosis of this degree of curve, we can start to consider how much it would've affected him. So, most people think he must've been in loads of pain with a curve like that. In fact, a lot of people with scoliosis don't get any pain in their back.
Once we created a 3D recreation of the spine, we can actually see that this was a spiral twist. That's very much what we see in modern patients today with scoliosis. The original photographs showed that he had a scoliosis which had about 70 to 80 degrees of curve which is quite significant. Using modern evidence from patients who have a scoliosis of this degree of curve, we can start to consider how much it would've affected him. So, most people think he must've been in loads of pain with a curve like that. In fact, a lot of people with scoliosis don't get any pain in their back.
The second thing to think about is his appearance. If you have a straight spine, you're going to be a bit taller and if your spine is twisted. And so, looking at modern patients, we can estimate that he would've been about 2 inches shorter than he would otherwise have been if he'd had a normal spine. However, we can see that the top and bottom of his spine were absolutely normal. So, those bones in his neck and in his lumber spine were fine. So, he wouldn't have had a tilt to his head. He would've had a normal, forward-looking head with normal neck movements. So, a lot of the things we see in Richard III's description by Shakespeare aren't borne out by the evidence either of the burial in the ground, or of the reconstruction that we've made. Shakespeare is clearly writing over a century after Richard III died and so, there's no way Shakespeare would've ever met Richard III and probably, never met anyone that had seen Richard III. So Shakespeare was right that Richard had a spinal deformity, but he was wrong in that he would probably not have had the limp that Shakespeare described. He probably wouldn't have had a significant hunchback. And similarly, there's no evidence for a withered arm as well. So, the bones of the arms and the legs, all look perfectly symmetric.
Graihagh - So, what's next on the cards with Richard III's remains? Are there any remaining mysteries to be solved?
Piers - Further research that is ongoing is looking at the weapon injuries that he has, looking at his genetics partially to see if he has any genes that might predispose scoliosis. But also, to look at other information we can tell from him. For example, certain genes tell you what colour eyes, hair and so on, an individual had.

24:52 - What is Alzheimer's?
What is Alzheimer's?
with Susie Hewer, Campaigner, Chris Dobson, Cambridge University, Jody Mason, Essex University
This week marked the 150th birthday of Alois Alzheimer - the man who first  described the disease he gave his name to over 100 years ago in 1906. The condition is one of a number of disorders that we collectively call dementia. These are progressive diseases that gradually rob sufferers of their mental faculties. They also become more common as we age. In countries like the UK, 1 in 6 people over the age of 80 are affected to some degree. But it also has a very profound effect on the people who care for victims of Alzheimer's.
described the disease he gave his name to over 100 years ago in 1906. The condition is one of a number of disorders that we collectively call dementia. These are progressive diseases that gradually rob sufferers of their mental faculties. They also become more common as we age. In countries like the UK, 1 in 6 people over the age of 80 are affected to some degree. But it also has a very profound effect on the people who care for victims of Alzheimer's.
Susie Hewer is a Guinness World Record holder for her extreme knitting. This year, she crocheted 139-metre long crochet chain while simultaneously running the London marathon. She did this to raise awareness for Alzheimer's after her mum Peggy developed the disease.
Susie - She started to get quite depressed. She was very withdrawn. The next phase was she started to have wandering incidents, because she'd lived with us and my husband worked at home, it hadn't been really a problem until then. But one day, he came downstairs, he found her sitting outside on the stairs because he wondered where she was and she said, "Well, I couldn't get in." he said, "Well, why didn't you ring the doorbell?" And she hadn't thought about that.
The next thing that sort of changed our lives dramatically was, I left her in the library while she chose a book. I went over the road to the cashpoint and came back again 5 minutes later, she wasn't there. So, I looked up and down the road, couldn't see her, went running one way and thankfully, I found her walking really fast with her head down. I said, "Mom, what's going on?" She said, "Well, you moved the car. I didn't know where you were, so I'm heading for home." She was heading in completely the wrong direction and the car was actually outside. So, at that point, we realised that the wandering could become a problem so I left my job to care for her full time.
For a while, it was sort of alright because we could go out together and we could go round gardens. Little by little these things disappeared from her and then we started to enter the really unpleasant phase. She didn't sleep at night at all. Then she became doubly incontinent and it's so humiliating for the person, but thankfully, by that stage, she was sort of beyond knowing what was going on.
With us this week to explain more about the causes of Alzheimer's disease and how scientists are trying to develop treatments to halt or reverse the disease are Chris Dobson from the Department of Chemistry at Cambridge University and Jody Mason a Biochemist at Essex University. They spoke to Chris Smith about what's actually going on in the brains of people who have Alzheimer's disease.
Chris D. - The fundamental thing that's happening is that our brain cells, our neurons are becoming damaged and ultimately dying.The result is that we find it more difficult to remember new things. We start as the disease progresses to begin to have difficulty in recalling the things we knew before. Ultimately, as you've heard from Susie's story, you begin to lose a great deal of control of daily activities and ultimately, you don't even recognise your friends and relatives.
Chris - What causes those nerve cells to die?
Chris D. - Well, we now know that it's caused by pathogenic agents. Pathogenic agents in this case are not bacteria or viruses from the outside world. They're actually species which develop from our own molecules particularly proteins. What happens is that these protein molecules and proteins I should say carry out every process that occurs in our body - so breathing, moving, eating, thinking - all are carried out by proteins. We have about 100,000 different proteins and they carry out all these functions because of their intricate structures. The way the one can think about these is proteins are made from series of building blocks called amino acids. The exact order of the building blocks determines which protein you make. This information is stored in our genomes, in our DNA. So, when proteins are made in the cell, they come out as long thin chains and to function, they have to fold up, into very specific, beautifully intricate structures and then they carry out their functions. But sometimes, these molecules which are packed together very closely in our cells so we can do fast chemistry and pass on messages, start to clump together. They misfold. They lose their correct structures and start to clump together. In the Alzheimer's disease, the primary protein that's involved or a fragment of protein is called the Abeta peptide. When this protein aggregates, it forms the familiar clumps and plaques in the disease. But actually, we now know it is not these plaques and such that cause the damage. It's a smaller aggregate, about the same size as viruses or bacteria that actually interact with the cells and move around and cause the disease to spread. We can understand how the disease kills, how the disease develops through cells dying and how this can then spread to other parts of the brain.
Chris - Jody, that Abeta peptide that Chris is talking about, where does that come from and what is it?
Jody - Well, the a-beta peptide is cut down. It's produced from a much, much bigger protein called the amyloid precursor protein. A couple of enzymes, molecular scissors come along and snip out this small section from the amyloid precursor protein. That produces this very small peptide. It's about 37 to 43 amino acids. It's that peptide that's incredibly sticky. It self-associates and then it forms into these very toxic amyloid plaques.
Chris - So, cells normally make the beta amyloid precursor protein. It's part of what your cells normally make as part of going about their cellular business in your brain. For some reason, a chunk of it gets chopped off which then is what starts to build up into these aggregates in the brain that ultimately lead to Alzheimer's.
Jody - That's correct and these amyloid plaques are exactly the type of - are exactly the things that Alois Alzheimer would've seen when he was looking down his microscopes 120 years ago.
Chris D - That's a very nice description. The interesting thing is that Alzheimer's is one a number of diseases that include Parkinson's disease, motor neuron disease, and even type 2 diabetes which were associated with these aggregation phenomenon of proteins. What we now know again is that our proteins all have a tendency to aggregate, but there are lots of protective mechanisms that come in. some of them are very motive words like molecular chaperons that look after our proteins and stop them making these improper interactions. But as we get older, these mechanisms start to get less efficient. And so, the molecules start to have a high risk of sticking together and once they stick together, then the process has started and progresses, and that's the onset of disease.
Chris - I was going to ask you about that because we read in the beginning that 1 in 6 people over the age of 80 is affected to some degree. But that means 5 out of 6 people Jody, aren't affected. So, what influences the likelihood of people having this big protein chopped up into these small bad bits of protein that then build up?
Jody - But as far as we know, it's a peptide that's being produced naturally throughout life expressed in all tissues. But for some reason - I mean, age is probably the biggest risk factor of all of course. As you live longer, you accumulate more of this peptide. For people with early onset Alzheimer's disease, they're producing more of this peptide. So, these scissors are coming in and cutting more often or there are mutations within the peptide itself that makes it more sticky, and therefore, more susceptible to aggregate, to stick together into these very toxic plaques. So, it's probably down chaperons not removing the peptide efficiently, but as I said, age is the biggest risk factor. So, as you get older, your risks of contracting Alzheimer's disease increases exponentially.
Chris - Anything else people can do Chris, to minimise their risk because there are some, it might be apocryphal claim that people in India have lower rates of Alzheimer's disease and some have suggested that it could be turmeric in the curry that they're fond of because turmeric is an anti-oxidant and it stops you burning out your brain cells, making these proteins that build up pathologically?
Chris D. - I think that there are a lot of very interesting ideas out there but actually, very few of them have got good statistical evidence. By far, the biggest risk factor as Jody says is age. It's really dramatic. At the age of 65, one or two people in a hundred will have symptoms of Alzheimer's disease. By the time you reach 85, it could be 1 in 3. Again, I think it's just chance that does this largely if you don't have particular genetic mutation.
Chris - And just to be clear, this is the loss of brain cells because these proteins are toxic to the brain cells and kill them off.
Chris D. - Absolutely. Once these aggregates start to accumulate and they could start perhaps 20 years before many of the symptoms begin then they start to kill the cells, and then it becomes a sort of chain reaction, a progressive reaction. But going back to your original comment, in terms of numbers, there are nearly a million people in Britain with Alzheimer's disease. There are over 5 million in the US. Worldwide, it's estimated that there are 40 million. More than half of those are in low and middle income countries. So, it's not just a disease associated with the affluent world because longevity is increasing everywhere. It's predicted in the next 35 or 40 years, that number will increase by at least a factor of 3.
34:14 - Cascading through the brain
Cascading through the brain
with Jody Mason, University of Essex, Gaz Bushell, Fayju
It's been estimated that one in two children growing up today, will live past 85 and so have a high risk of developing Alzheimer's. In order to educate students about the disease which could make a major impact on their lives, Jodie Mason from the University of Essex has teamed up with a games developer to make a video game which he hopes can communicate the complicated biochemical pathways involved. He explained more to Hannah Critchlow
so have a high risk of developing Alzheimer's. In order to educate students about the disease which could make a major impact on their lives, Jodie Mason from the University of Essex has teamed up with a games developer to make a video game which he hopes can communicate the complicated biochemical pathways involved. He explained more to Hannah Critchlow
Jody - So, we've created a game called Cascade and I've teamed up with a chap called Gaz Bushell from Fayju, a company that make computer games. The idea is that we want to educate really anybody who plays computer games, but of course a big demographic is young people, teenagers, who access these things. In Cascade, you actually step inside the brain of somebody with Alzheimer's disease so you can look around you, you can see the neurons, these brain cells all around you. You can see the amyloid precursor protein and the enzymes, these molecular scissors that come in and snip out that peptide segment, and you can watch that toxic self-association, that clumping to form these amyloid plaques. Because it has this sort of intergalactic feel, the only real artistic license that we've taken is that the player is controlling a spaceship. But a lot of the things that that spaceship does really mirrors the therapeutic intervention strategies that labs like my own at Essex and Chris's over in Cambridge and drugs companies are trying to do so, for example, to block those scissors and stop them cutting APP or if the beta-amyloid peptide does get produced to stop it from self-associating - so somehow detoxify it or stop it clumping.
Hannah - That's an innovative way of trying to switch people on to trying to understand this really complicated biological process that will probably affect them in the future. Well earlier this week, we took Cascade for a test drive. Kate Lamble accompanied Gaz Bushell, the games developer into Coleridge Community College to see what a class of 13-year-old students made of it.
Gaz - Cascade is a game where you fly around this infinite landscape effectively from brain cell to brain cell, fighting this malevolent force which is destroying everything in its path. And that force happens to be Alzheimer's disease.
Kate - Cascade is a game with a difference. Players control a spaceship which flies around a universe of brain cells, trying to protect them from attackers. As Gaz explained, it's all based on the actual science of Alzheimer's.
Gaz - Jody was explaining the amyloid cascade hypothesis to me in kind of layman's terms I guess. Being a programmer, the more that he would tell me about these aspects of the biochemistry involved in what was going on in Alzheimer's disease, the more I could see each of those individual elements would breakdown into a game component.
Student - Okay, so what am I aiming for?
Gaz - So, you see that giant orange thing?
Student - Yeah.
Gaz - That's a plaque. So you want to go to the source of that to try and stop it from forming.
Student - And you just fly around and you'll find the blue things.
Kate - So, the attackers represent the toxic sticky beta amyloid protein which can build up and disrupt the brain in Alzheimer's. Players break them down, keeping the brain cells healthy and delaying the onset of the disease just like drugs are trying to do in real patients at the moment.
Gaz - It's the same as most games. You're generally fighting something. We might as well make it so that you're fighting something very real. Through that process of engagement with it, you can understand more about the science but also, have an engaging and fun interactive experience.
Jacob - My name is Jacob and I'm 13 and I'm from Cambridge. I just had a go. It's stunning. It's out of this world, how good it is.
Kate - We tested the game on the newly released oculus system which is essentially a virtual reality headset that gives you a 3D immersive gaming experience. The novelty of the system might have contributed to some of the student's excitement.
Student - Does anyone want a go now?
Student - No, guys - it's in a line.
Kate - But the game is also being released for free on the 2D Ouya system. Then Gaz and Jody hope it will launch on major platforms like the PlayStation and Nintendo.
Student - The concept of the game looks pretty cool.
Student - It's like really cool.
Julian - I'm Julian. I'm 13 and I'm from Canada. It's just so breath-taking and I can imagine a lot of games being adapted to this.
Kate - By raising the public's awareness of Alzheimer's particularly with the young, Gaz and Jody hope that more can be achieved in terms of care, empathy, and funding. But does this approach actually work? The students we met certainly seem to be enthusiastic about gaming. But when asked what they learned, admittedly, only after a few minutes on the system, they didn't seem to have grasped what Alzheimer's was quite yet.
Student - Brain tissue in your head starts to deteriorate. I don't really know. I think I know some who has it.
Student - So, where's the gas cloud? There it is.
Kate - So, is it really an educational tool? I asked their teacher for her thoughts
Hillary - I'm Hilary Tunnicliffe. I'm a science teacher at Parkside Federation Academies on the Coleridge campus. I have rarely seen some of the year 8's as excited as they are about the game that you've just shown them. The virtual reality headset has inspired those children. I think anything like this which will inspire this sort of enthusiasm is a great teaching tool because they get interested in it. But actually, as the game is going on, they're beginning to work out what they're doing and then linking that with the stuff that I would then be teaching them. My family have had some personal experience of Alzheimer's. It's a very misunderstood condition and yet, it's going to affect more and more people with an ageing population. Anything that can be done to actually increase, not just the knowledge of it, but the awareness of it can only be a good thing.
If you want to have a go on Cascade, you can download it completely for free at www.ouya.tv/games.
40:33 - Finding the right drug for Alzheimer's
Finding the right drug for Alzheimer's
with Chris Dobson, University of Cambridge
In the drive to find a drug that prevents Alzheimer's disease, scientists are using fruit flies as a model organism to screen various compounds that could one day lead to a cure. Hannah Critchlow spoke to Cambridge neuroscientist Chris Dobson to find out more.
flies as a model organism to screen various compounds that could one day lead to a cure. Hannah Critchlow spoke to Cambridge neuroscientist Chris Dobson to find out more.
Chris D. - I've been described as many things. I mean, our view is that many of the diseases in the past or all of the diseases I think in the past really, until you understand the real mechanism, it's almost impossible to develop effective drugs. I think this has been one of the problems so far in trying to treat Alzheimer's disease. So, what we're trying to do in fact is to take the Abeta peptide that Jody mentioned and understand exactly the mechanism by which it begins to clump together the steps that it goes through, how it forms species that are toxic, why they're toxic, and how it eventually forms to plaques. And we found a lot out about that and we've done that in the test tube. And so, what we want to do now and what we're beginning to do now is to say, "Does the same sort of thing happen in living systems?" We've taken very simple organisms. You're going to ask me about the fruit fly which is one of the ones...
Hannah - I am about to. I've heard rumours that you work with the common garden fruit fly that maybe hover above people's bananas as they get a little bit mouldy.
Chris D. - We do. I hope these ones don't hover around people's bananas, but what we're really trying to do there is actually to generate what we sometimes call test tubes with wings that we can put via transgenic means by genetic engineering the same peptide - the Abeta peptide - into the brains of fruit flies. And then we can choose lines of flies which have much the same sort of symptoms as you see in Alzheimer's disease. They form plaques, they have difficulty moving around. We haven't checked very carefully what they can remember, but we know that they actually don't live so long and so forth. That gives us a system where we can look at thousands or millions of flies to get statistics on the way that molecules interact with the Abeta peptide. And so, what we're able to do is to take molecules we found in the test tube and see what effect they have in these model organisms.
Hannah - And so, you are able to really screen new compounds or compounds that are in existence at the moment.
Chris D. - Absolutely.
Hannah - And find out if they can revert this misfolding of this protein that's toxic to nerve cells and stop it from causing Alzheimer's.
Chris D. - Absolutely and I think there are really two aims that we would have. One is to reduce the risk of it, the chance of this happening which of course would be a bit like a sort of statin for heart disease, it just makes it less likely you'll get it. The other is actually, to try to do therapeutic, a real cure. Well at least, to find a molecule that will stop this process occuring so the disease doesn't progress. So, we're able to test these ideas out in these simple systems.
Hannah - I mean, the drugs that are currently used for those people that have Alzheimer's, what do they do? Do they not affect all these protein misfolding and try and revert that?
Chris D. - Well actually, what they don't do is actually slow down at all the progression of disease. There's no evidence that any current drugs actually have an effect on the progression of disease I'm afraid. What drugs do do is how to alleviate some of the symptoms. And so, that's really all we can do for patients at the moment. But I hope of course that we can find drugs that will actually supress the processes that give rise to a disease.
Hannah - And that's why it's so imperative that you continue your work looking at the chemistry, this basic biology in trying to develop new compounds.
Chris D. - Absolutely and I think we heard from some young people. I think they're the people who need to be aware of what's going on because it takes a long time to develop drugs. The amount we spend on research in Alzheimer's disease is minute compared to the amount we spend on cancer and heart disease even though the cost of society and economy are much greater in Alzheimer's disease.
Hannah - Jody.
Jody - Yeah, just following on from that point really, it costs the UK economy 23 billion pounds a year. It's just huge and with the numbers going through the roof, that's only going to get a lot worse. You know, it's set to hit a million and then start doubling every sort of 25 years, and I mean, with the game in particular, we're trying to get young people interested in an area that they probably don't think is going to affect them. But the young people of today are going to become the carers of tomorrow.
Hannah - I suppose previously there was quite a lot of stigma around Alzheimer's disease as there was with cancer. So patients that had cancer 50 years ago, they didn't want to mention the C word.
Chris D. - I think it's absolutely right. 50 years ago, if someone was diagnosed with cancer, the question was, "Did you tell them?" And the answer is, you didn't normally tell them because it was almost nothing you can do. I think the developments in the 1970s, particularly in 1971 National Cancer Act in the US opened up the whole field and people started talking about it, therapists started appearing, a lot more money was put into it, a lot more people are engaged in it, and that's absolutely what we need to do now. So, these methods that Jody is talking about, to get people aware of the disease, and aware that we can do something. I'm very optimistic. I think we can have dramatic effects on the onset of disease and prevention of disease, but it's going to take a long time. It's going to take a lot of effort just as cancer did.
45:40 - Using arm hair to grow brain cells
Using arm hair to grow brain cells
with Richard Wade-Martins, University of Oxford
Alzheimer's Disease is a little-understood neurodegenerative disorder and current treatments only help with the symptoms. However, Dr Richard Wade-Martins and his colleagues have taken an important step, which will enable scientists to test drugs on petri dish-grown brain cells. To find out more, Chris Smith and Hannah Critchlow spoke to Dr Richard Wade-Martins from Oxford University.
Richard - The way this works is we have patients in the clinic that we study in great detail. We understand they have Alzheimer's, we may even understand they have a subtype of Alzheimer's with particular clinical features. So what we can then do is we can take a skin sample from these patients. Some groups will take hair plucks as you've heard. Other groups will take skin punch biopsy. So a skin punch biopsy is where you take a small piece of skin, about the same size as you'd punch out a piece of paper from A4 paper using a hole punch. So, you take this skin sample from somebody. You bring it back to the laboratory and you chop up the bit of skin into little pieces and you put it in a plastic tissue culture dish surrounded by a liquid medium the cells like to live in and these skin cells will sink to the bottom of your plastic dish and they'll grow, and over 2 or 3 weeks, they'll fill the bottom of your plastic dish.
Now, you have skin cells. What we can do - based on some work that was originally developed by a chap called Shinya Yamanaka in Japan in 2006, he won the Nobel Prize for it in 2012 - is we can turn those skin cells into stem cells. So, we use viruses that make these special genes called reprogramming factors. That's 4 reprogramming factors you need. You can tweak these skin cells and they become stem cells. Over a period of weeks and months, you can grow these stem cells in the laboratory and once you've got stem cells, you can make any cell type you like.
When you think about these neurodegenerative diseases like Alzheimer's and Parkinson's, and motor neuron disease, it's a particular type of brain cell that dies off. In Alzheimer's, it's what's called cortical neurons. It's the neurons that make the part of your brain at the front and the top. Brain cells that make you think and remember. We can make these types of neurons in a dish, grow them out and over a period - it takes 100 days to turn these stem cells into these brain cells. And then we can study them and start to understand what's different about these brain cells from patients and from controls from healthy people.
Chris - And equally, I suppose Richard, the other benefit of doing this is that you've got real human nerve cells to study but also, you can take some of the chemicals that Chris is trying to develop to affect this folding or misfolding process and ask, how does this affect the way that these human nerve cells behave, which means you're sort of a step up in the clinical testing of these agents.
Richard - Absolutely and you're working with human brain cells which is so important. This stem cell technology is having a revolutionary effect right across medicine. But I think in neuronal diseases and diseases of the brain, it's particularly important and powerful because you can't drill a hole in somebody's head and take out some neurons to study. You have to make them as best as you can in a dish. Once, we've grown them in the dish, people have shown that these nerve cells from Alzheimer's patients have some of these protein problems if you like that we've talked about. They accumulate this Abeta peptide. They accumulate these tangles, these aggregates inside the neurons and start to kill the nerve cells off - both in the brain but also in the dish. And then you can take therapies that we know work in the clinic and test them on our dish and see if they have an effect on the nerve cells that we've got. But then you enter the world of testing and screening for new compounds and you're actually using brain cells from patients with Alzheimer's to test, to screen, to look for new compounds. It's a very powerful, exciting technique. It's exciting time in the field.
Chris - Can you also ask other questions such as, how do the nerve cells respond to these pathological proteins building up? Does it stop talking to other cells as efficiently as it would've done before which may explain some of the features of Alzheimer's disease and people who are affected?
Richard - Alzheimer's view of Alzheimer's was that these neurons are dying. But actually, we think decades before the neurons die, they stop working properly. They stop talking to each other. So, the brain is a network of neurons in constant communication. The idea is maybe 10, 20, 30 years before the neurons die, they stop talking to each other. If we can understand how they stop talking to each other decades before the disease, we can enter the era of predictive and preventative therapies. We've talked about two particular pathologies, types of protein aggregates you find in the brain. There's Abeta peptide and there's also a tau protein. In my own work funded by the Alzheimer's Society, we're taking these brain cells and we're deliberately deleting the tau protein from the genome from the chromosomes of those cells, and then seeing what effect does the Abeta peptide have on these cells. So, if you manipulate one of these two proteins, do you make the disease less severe at least in your cell culture model?
Chris - Lastly, just very briefly Richard, may - what you're doing also - hold the key to rescuing the affected brain in Alzheimer's? In other words, if we can make new nerve cells from skin stem cells, we could give people their own brain cells back to perhaps makeup the deficit of cells they've lost in the disease and therefore, help them to remain healthy for longer.
Richard - So ultimately, that may be possible. So, that's a dream for many of these neurodegenerative diseases such as Parkinson's and motor neuron disease, and also Alzheimer's but it's really difficult. You've got a loss of brain cells right across the brain, particularly in the case of Alzheimer's. So, cell transplantation therapy may be possible, but I think most scientists would probably think that if we can work out why the disease occurs, and to prevent it happening, to holt it early on, that's probably going to be more realistically beneficial than transplanting neurons which is possible but fraught with technical difficulties.
Hannah - We've heard on Twitter that how come it takes a lifetime for someone to develop Alzheimer's and show the symptoms, but when you have your cells in a dish, they can develop Alzheimer's within a hundred days of their lifespan.
Richard - I suppose it depends what you mean by developing Alzheimer's. So, when we say that a patient develops Alzheimer's say, at the age of 70, there is some evidence from brain imaging studies and other studies that actually, things may be going wrong in your brain at the age of 25. So, it may be that actually, neuronal changes happen much, much earlier, decades before somebody will actually show Alzheimer's disease. So, when I say that in the dish, cells develop signs of Alzheimer's disease, what I mean is they show some changes in the way that critical proteins such as Abeta peptide and tau are regulated in how they function. So, when people make stem cells from patients with Alzheimer's, they typically take individuals who have Alzheimer's for genetic reasons. They have a genetic mutation in a gene which leads to Alzheimer's disease at a young age - say, about the age of 40, 45. So, these individuals are getting Alzheimer's earlier and it's genetically inherited. And then once you made the stem cells and differentiate these cells into neurons, you wait 100 days, you see molecular and biochemical changes in the way that Abeta peptide is produced and the way that tau is modified. Particularly the type of modification called phosphorylation where phosphate group is added on to tau. So, these biochemical changes of elevated Abeta peptide and tau phosphorylation can be seen in these neurons in these patients after 100 days. But I think it's a little bit shorter saying these neurons actually have Alzheimer's after 100 days.
Chris - Chris.
Chris D. - I think this again, it's a very, very interesting question and one that isn't really understood in detail. But I think that our sort of feeling about this disease in general is that proteins all have a tendency to aggregate. So, they're folding and coming to form these wonderful structures to do their function. But at the same time, there's a certain fraction in which they don't fold properly and so, they aggregate. The reason that we don't see the effects of these until old age - we think - is because there are all these protective mechanisms that come in, particularly these molecular chaperones that try and look after these naughty proteins that are trying to get together. And the effect is that they inhibit these processes. I think that it's now becoming clear that there are a lot of very specialised chaperones that are not just within the cells, within the neurons, but actually, also outside the cells. There may be even effects coming from different types of cells which affect neurons. So, I think the situation within the intact brain, particularly the protective mechanisms that exist may actually differ in the sort of timescale when this regulatory control is lost from that which happens with an individual cell. But again, it's a really fascinating point and some of these are absolutely crucial that we understand.
Chris - So, some of these treatments that you're trying to develop may not revolve purely around stopping the misfolding of the protein in so much as targeting the protein itself. You could actually be providing new chaperone proteins or similar molecules that do the sort of Alzheimer's equivalent of a leg brace and someone with a weak leg. They prop the proteins in the right shape so they can't misfold in this way.
Richard - Well, it's absolutely right. There are potential, almost artificial chaperones if you like. One type is antibodies. Antibodies are proteins that bind to a very specific molecules and if you can get them to bind to the molecules which are likely to aggregate then they have much the same effect as chaperones of inhibiting the proteins sticking together.
Chris - But the key question we must finish on is by asking you, how long before we get to a stage where we have something tangible to offer people rather than just as you were saying with Hannah that at the moment, we only have drugs that make the symptoms a bit more tolerable. We don't have anything that stops the disease process.
Richard - I think that conventional drug takes 10, 15 years to develop. I think one has to put in perspective to the fact that bacterial viral diseases, people have been working for 200 or 300 years to understand the origins of disease.
Serious research on Alzheimer's disease has only really been going for 30 years. So, we're very early stages. But there are hopes that there could be some breakthroughs. I think personally, we'll find a cocktail, a whole range of different drugs that might be effective on different people or in combination. And some of these drugs may already be in existence for other conditions and this is always one of the hopes in drug discovery that you'll find a compound that does something, developed to do something, but actually, have some other effects. So, many people are looking at molecules which are already authorised for usage as drugs that perhaps just might have effects in Alzheimer's disease. There are classic examples of this. One of them for example is amitriptyline which is used in pain control. It was developed first as an anti-depressant. But in much, much lower doses, it is very effective in pain control. So, we might find these treatments more rapidly. But I think Alzheimer's disease, dementia is going to be like cancer. It's going to be a process of attrition. We'll start to find treatments that begin to be effective and then we'll get better and better at doing it. But I think it will be like cancer or heart disease. It would be a long struggle.
Chris - Jody.
Jody - The only thing I would add is, the other thing we don't have at present is a good biomarker where you don't have any real way of saying that somebody absolutely had Alzheimer's until after they pass away and then you look at the brain. So, the other thing, I mean, Richard touched on earlier which is a lot of the damage is already done by the time those clinical features start to present themselves. So, I think when you do, if and when you do find this sort of wonder drug, you're going to have to get it in pretty early on, maybe a couple of decades before any of the symptoms are there.
Chris D - Perhaps I could add something to that. One of the big challenges again in Alzheimer's disease is this biomarker problem. It's not just for diagnoses. It's that if you want to develop a drug, you want to know if it works. To know if it works, you really would like to know in less than 10 years if it works. I think that there are lot of efforts now going on to find biochemical markers of the disease which you could ideally do with a blood test or something like this where you can actually tell...
Chris - But that's presumably where your work comes in, Richard because that's sort of what you're able to do. You can look at these cells and ask, do they change their behaviour and responds to compounds like Chris is developing when you chuck them on them.
Richard - Yes, it's not really a biomarker though. I mean, the biomarker point is essential for two reasons. First of all, you need to be able to identify these patients before they become patients. Chris' comparison with statins is absolutely right. You need a biomarker to detect elevated risk factors such as cholesterol in cardiovascular disease and then a therapy which reduces those risk markers which is a preventative treatment, much earlier than a treatment treatment to cure people once they're real.

58:18 - Do brains get tired like muscles do?
Do brains get tired like muscles do?
Hannah - So, that feeling of fogginess that comes with brain fatigue. You've probably experienced it at some point. Scientists haven't yet figured out the exact biochemical pathway leading to it, but it seems to be linked to, perhaps unsurprisingly, how much you use your brain and how much fuel it has available. Here's Dr. Catherine Hall, brain energy expert from University College London explaining what happens when muscles in your body get tired from too much exercise.
Catherine - When we've been exercising, lactic acid does build up in our muscle. But actually, it probably doesn't cause the pain and muscle fatigue we feel after exercising. Instead that is caused by a shortage of fuel and a build-up of lots of different molecules that are by-products of muscle activation. Lactic acid may be one of these molecules or it may actually reduce muscle tiredness. In our brains, lactic acid also builds up when nerve cells are active. But nerve cells can use lactic acid as a fuel. So, while it might be a sign of an active brain, if anything it should stop our brains from being tired.
Hannah - Aha! So, activating our brains actually provides a short burst of lactic acid which is an actual fuel for our brain. So, that shouldn't cause fogginess or brain fatigue. But surely, this fuel burst will wear out at some point.
Catherine - We do get tired though and it turns out, a good dose of glucose does boost brain performance. And handily, eating some glucose it particularly good at boosting our ability to do tasks that use verbal memory so it'll especially help you to do your French homework. You have to be careful though as too much glucose will stop your brain working so well. By eating 38 M&Ms before starting your homework, you should get a brain boost that will last you an hour or so. Watch out though. Your brain isn't actually burning all that extra energy so French homework won't stop you getting fat.
Hannah - Thank you, Catherine. She also adds that she doesn't want to be responsible for everybody reaching for the candy and worldwide surge in type 2 diabetes with this answer. She does suggest that healthier, slow release glucose treats, like maybe a banana, could also boost brain power, or you could just take a break to refocus your mind.









Comments
Add a comment