Volcanic pollution, the Ozone Hole and the Greenhouse Effect - The Atmosphere Show
This week, scientists recreate hair follicles, we uncover a means of making hydrogen in a hurry, hear about a stealthy way to destroy cancer and find out why a dose of herpes could be good for you. Also, John Grattan describes the biggest atmospheric pollution event in history, we discover with Rod Jones the role of water in the greenhouse effect, and Jonathan Shanklin tell us the 'hole' story of the ozone layer. Plus, in Kitchen Science, we make a cloud in a bottle!
In this episode

Al-uminating answer to fuel cell problem
US scientists have stumbled on a safe way to make large amounts of hydrogen, on-demand, to fuel environmentally-friendly vehicles. Jerry Woodall, from Purdue University, has found that an alloy made by mixing gallium and aluminium can be reacted with water to produce large amounts of hydrogen gas, which can be collected and fed to an engine. During the reaction only the aluminium is used up; it turns into aluminium oxide which, together with the gallium, which is not consumed, can be recycled.
The trick works because normally aluminium won't willingly react with water because the metal forms a protective oxide "skin" on its surface. But the gallium interferes with the formation of the oxide layer, enabling the aluminium to react freely with the water. The two metals are melted together to produce the alloy, which is then turned into pellets for the reaction with water.
The benefit of hydrogen-powered vehicles is that they are very clean since the only exhaust product is water. And if energy used to recycle the aluminium oxide back to aluminium came from non-fossil sources the process could even be carbon-neutral, and feasible too. "A mid-sized car with a full tank of aluminium-gallium pellets could take a 350 mile trip and it would cost US$60", Woodhall points out.
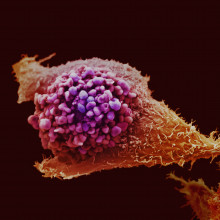
Killing cancer by stealth
Cancer Research UK-funded scientists at the Institute of Cancer Research in Surrey have been testing a new way to kill bowel cancer cells with a kind of "smart bomb". The technology is called GDEPT, which stands for Gene-Directed Enzyme Prodrug Therapy, and it's based on a rather clever virus, and a harmless chemical that goes by the catchy name ZD2767P, known as a prodrug. 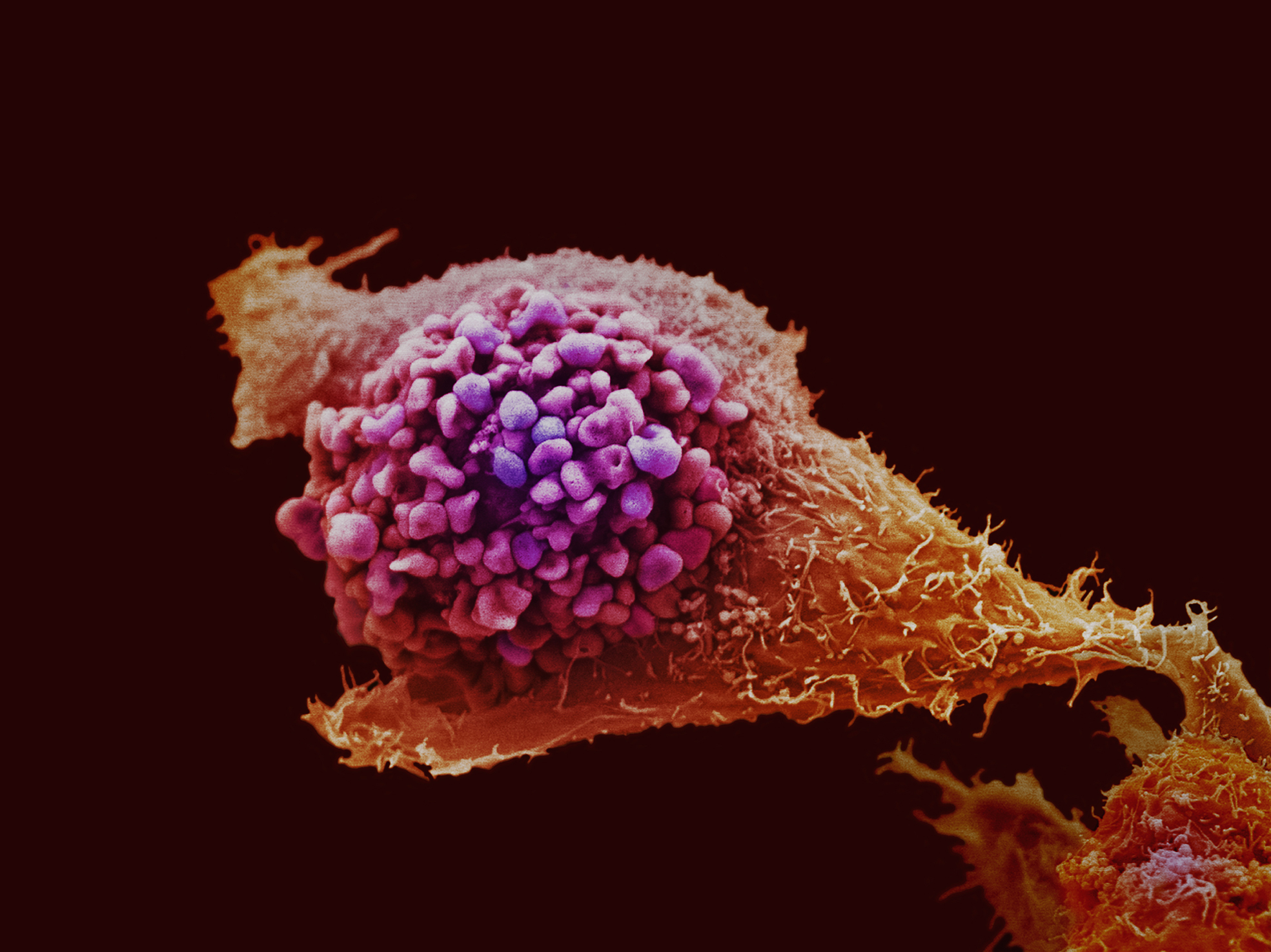
The scientists, led by Professor Caroline Springer, have engineered a harmless cold virus to contain an enzyme called Carboxypeptidase G2, or CPG2. The enzyme is only activated when the virus infects cancer cells, because it's designed to respond to telomerase - a protein that makes around 8 out of ten cancers immortal. Then when the cells are given the prodrug, the CPG2 enzyme coverts it to a powerful cancer-killing drug. The scientists tested their technique in bowel cancer cells grown in the lab, then in mice with bowel tumours. They found that GDEPT could double survival in mice that had been treated, compared to those that hadn't. In a double-whammy, the researchers also found that dying cancer cells produced signals that killed neighbouring cancer cells, increasing the effectiveness of the treatment.
And because healthy cells don't have telomerase, they don't activate the enzyme, so they don't make the killer drug.
Current cancer treatments often damage healthy cells as well as cancer cells, causing unpleasant side effects. But targeted treatments like GDEPT should be more specific for cancer, reducing side effects. It still needs more work before it's suitable for clinical trials in cancer patients, but GDEPT could prove to be a useful therapy for cancer in the future.
The virus that came in from the cold
US researchers have performed the microbiological equivalent of fighting fire with fire by showing that animals infected with members of the herpes virus family are much better at fighting off infections caused by virulent bacteria than uninfected animals. Writing in this week's Nature, Skip Virgin and his team at Washington University in St Louis infected mice with one of two herpes viruses, either MHV-68 or mouse CMV, both of which cause the rodent equivalent of human glandular-fever-like illnesses.
After the initial infection had passed, the animals were then challenged with potentially lethal doses of either Listeria, or a second bug known as Yersinia pestis, which causes bubonic plague. Compared with a group of control animals, the virally-infected mice were all highly resistant to the bacteria and the team found higher levels of at least two immune-boosting hormones, called interferon-gamma (IFNg) and TNF-alpha, in their bloodstreams.
"This suggests that the viral infection is in some way beneficially modifying the immune system of the host," says Virgin. "A key feature of the herpes viruses is that, after infection, they remain in the body for the life time of the host, a state known as latency. It therefore stands to reason that the virus should want to protect its host, because if the host dies the virus dies".
As yet the team are unsure if the effect also exists in humans, and they're now exploring whether the protective effect extends to defending animals against other viruses and whether it will be possible to re-create the effect without having to be infected with the virus - perhaps by means of some kind of vaccine.
What’s the matter?
Physicists at the University of Pittsburgh have created a new form of matter, which they published in the journal Science this week. Unfortunately it's not a form of chocolate that contains zero calories. The new matter is called a polariton superfluid, which combines the characteristics of laser light with the world's best electrical superconductors. To create the new substance, the scientists filled a solid nanoparticle structure with tiny energy particles called polaritons. 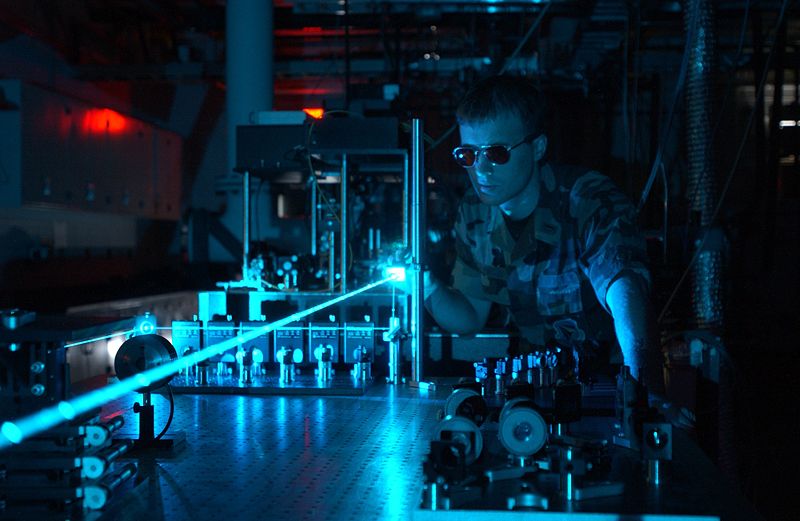
By trapping the polaritons in a tiny "cage", it slows them down. This means they come together to act in a co-ordinated way as a single energy wave, rather than a load of particles bouncing about. The wave produces light, similar to laser light, but using much less energy. The researchers also found that energy moved around from one point to another within the superfluid, similar to the situation seen in superconductors - which are highly efficient electrical conductors.
Normally experiments like this have to be done at extremely low temperatures, because superfluids and superconductors are very unstable. But the new polariton superfluid seems to be much more stable. In fact, the lead scientist Professor David Snoke thinks he might be able to create the new matter at room temperature.
At the moment, this is all fairly esoteric physics, but in the future, the technique could be used to transfer light through solid matter. This might be useful for building super-fast computers, or improving communication technologies.

13:58 - A Cure for Baldness?
A Cure for Baldness?
with Professor George Cotsarelis, University of Pennsylvania
This week, there was some good news for the follicularly challenged, because researchers at the University of Pennsylvania have taken the first steps towards discovering a cure for baldness. They noticed that when wounded skin was healing, some of the cells in the upper layer of the skin, the epidermis, turned into hair follicles. By looking at the genes that were turned on in those cells they were able to isolate a particular genetic pathway that lets hair follicles form during healing in the same way that they did during embryonic development. Chris Smith spoke to Professor George Cotsarelis: George - The dogma has been that hair follicles do not develop during adulthood, so once you lose a hair follicle, the prevailing wisdom has been that you cannot regenerate it. So, we were surprised to find that actually when we wounded mice, during the healing process we noticed new hair follicles forming in the centre of the wound. On close examination we realised that these follicles were undergoing very similar changes that are found when the hair follicle first develops in the embryo.Chris - Now, when you say you wounded the skin, do you mean you actually removed a full-thickness piece of skin or just the surface, what did you take away?George - We actually removed the entire skin, a full thickness on the back of the mouse and allowed it to heal.Chris - So, if you made a full-thickness injury to the skin that means that the regeneration of those hair follicles, the cells that are doing that must be coming from somewhere else, it is not some deep-rooted stem cell coming in from below the lesion? So, where are those cells coming from?George - We were first expecting to find new hair follicles to be coming from the stem cells that are actually present in the hair follicles at the periphery of the wound. We were quite surprised to find that, although the stem cells at the periphery of the wound contributed to wound healing, these cells did not contribute to the new hair follicles. What we actually found was that the hair follicles developed from cells in the epidermis and from the upper part of the follicle.Chris - So, what sort of cells are they then, if they are not follicle cells, what is actually producing the follicles?George - The epidermal cells that are creating these follicles are essentially reprogrammed and instructed to make a new hair follicle. So, the signals that go between the epidermis and the dermis during the neonatal time period or during embryogenesis are re-utilised in the adult to regenerate new hair follicles on the face of wounding.Chris - Do you know what the factors are which are provoking those stem cells which are in the skin, because presumably that might be quite an interesting pattern of genes to know about if we want to try and recreate hair follicles in aged men?George - We know from developmental biologists who have been working on hair-follicle development that the gene beta-catenin, which is in the Wnt pathway, is very important for normal hair-follicle development. So, we looked at beta- catenin and it was being expressed in these new follicles, and we also then blocked beta-catenin after the wound had closed. If you block beta-catenin at that point, then no new hair follicles will form but --perhaps more exciting than that -- when we activated the Wnt pathway, we found an increase in the number of new hair follicles that formed, in fact the number doubled. So this indicates that during this wound-healing process, these cells are susceptible to manipulation by signals that are known to be important for hair-follicle development. So, we think that in the future we will be able to design treatments based around the response that we see after wounding.
George - The dogma has been that hair follicles do not develop during adulthood, so once you lose a hair follicle, the prevailing wisdom has been that you cannot regenerate it. So, we were surprised to find that actually when we wounded mice, during the healing process we noticed new hair follicles forming in the centre of the wound. On close examination we realised that these follicles were undergoing very similar changes that are found when the hair follicle first develops in the embryo.Chris - Now, when you say you wounded the skin, do you mean you actually removed a full-thickness piece of skin or just the surface, what did you take away?George - We actually removed the entire skin, a full thickness on the back of the mouse and allowed it to heal.Chris - So, if you made a full-thickness injury to the skin that means that the regeneration of those hair follicles, the cells that are doing that must be coming from somewhere else, it is not some deep-rooted stem cell coming in from below the lesion? So, where are those cells coming from?George - We were first expecting to find new hair follicles to be coming from the stem cells that are actually present in the hair follicles at the periphery of the wound. We were quite surprised to find that, although the stem cells at the periphery of the wound contributed to wound healing, these cells did not contribute to the new hair follicles. What we actually found was that the hair follicles developed from cells in the epidermis and from the upper part of the follicle.Chris - So, what sort of cells are they then, if they are not follicle cells, what is actually producing the follicles?George - The epidermal cells that are creating these follicles are essentially reprogrammed and instructed to make a new hair follicle. So, the signals that go between the epidermis and the dermis during the neonatal time period or during embryogenesis are re-utilised in the adult to regenerate new hair follicles on the face of wounding.Chris - Do you know what the factors are which are provoking those stem cells which are in the skin, because presumably that might be quite an interesting pattern of genes to know about if we want to try and recreate hair follicles in aged men?George - We know from developmental biologists who have been working on hair-follicle development that the gene beta-catenin, which is in the Wnt pathway, is very important for normal hair-follicle development. So, we looked at beta- catenin and it was being expressed in these new follicles, and we also then blocked beta-catenin after the wound had closed. If you block beta-catenin at that point, then no new hair follicles will form but --perhaps more exciting than that -- when we activated the Wnt pathway, we found an increase in the number of new hair follicles that formed, in fact the number doubled. So this indicates that during this wound-healing process, these cells are susceptible to manipulation by signals that are known to be important for hair-follicle development. So, we think that in the future we will be able to design treatments based around the response that we see after wounding.

17:58 - Science Update: The Atmosphere
Science Update: The Atmosphere
with Chelsea Wald and Bob Hirshon
Bob - This week for the Naked Scientists, we're going to focus on the water in our atmosphere. I'm going to talk about how scientists are improving their ability to predict the intensity of hurricanes, but first, Chelsea's here to tell us why some mountaintops may not be getting their share of H2O.
Chelsea - Air pollution from cities can deprive nearby hills and mountains of rainfall. This according to meteorologist Daniel Rosenfeld of Hebrew University in Jerusalem. Looking at fifty years of data from a mountaintop in China, he and his colleagues found that while air pollution has steadily increased, rainfall has decreased.
Daniel Rosenfeld (Hebrew University, Jerusalem): Not only that, but when we look into the day to day variability in the amount of rain over the mountain, it is going together with the day to day variability in pollution.
Chelsea - Normally, moist air deflects up a mountainside, then cools and condenses into rain over the mountaintop. But if that moisture condenses on air pollutants, the drops don't get big enough to fall. Rosenfeld says this effect could dry up alpine watersheds, causing problems for the very cities creating the pollution.
Bob - Thanks, Chelsea. And while hills and mountains may have the problem of too little rain, some costal areas have had the problem of too much--in the form of hurricanes. In recent decades, scientists have become much better at predicting the paths of hurricanes, but not at predicting sudden changes in their intensity. That's why University of Washington atmospheric scientist Robert Houze and his colleagues are studying a part of the hurricane called the "eyewall": the strong winds around the calm eye of the storm. Howze says those winds gain momentum as the eyewall contracts. But eventually the eyewall collapses, and is replaced by a new one that's wider and weaker.
Robert Houze (University Of Washington): And this has a great deal to do with the intensity of the storm as a whole; the wider the eyewall, usually the less intense the winds.
Bob - Using data from planes flown into the eyes of hurricanes, his team is learning more about the eyewall cycle. The data will help create better models for predicting a storm's dramatic fluctuations.
Chelsea - Thanks, Bob. Next time, we'll talk about what scientists are learning about disorders of the brain, including Alzheimer's, depression, and schizophrenia. Until then, I'm Chelsea Wald...
Bob - ...and I'm Bob Hirshon, for AAAS, The Science Society. Back to you, Naked Scientists...

Is rainwater clean enough to drink?
It depends on where you are. Everyone assumes that clouds are sterile, but scientists have recently discovered that clouds contain a species of bacteria called Pseudomonas. These bacteria live in the air and seem to use clouds as a way of transporting themselves. It's able to do this because it has a way of causing ice nucleation - It's got certain chemicals on it's surface that makes tiny ice crystals form, and this makes the cloud form ice crystals around the bacterium. This makes it heavier, and so it flutters down to earth - using the clouds and winds as a transport mechanism. These bacteria don't seem to cause any harm to humans though.
What can harm you are the other chemicals that are dissolved in the water as the rain falls down to earth. If you're isolated from pollution sources, the rain is coning from a pristine ocean and will be pretty clean. If you're in a built up area, or downwind of heavy industry, power stations etc, these things can be pumping out all sorts of chemicals - particulates, carcinogens, dioxins and even heavy metals. These particles get into clouds and encourage the clouds to form water droplets, falling as rain.
It's also interesting to note that some of the dust that rain deposits on your car has come all the way from the Sahara desert. Dust from the Sahara gets blown high into the atmosphere and is distributed accross the globe.
Is there any use for lime scale?
The lime scale is basically calcium carbonate, so it could be compressed together to make chalk! Calcium carbonate is also used in paint, ceramics, as the starting ingredient for builders lime, in nappies, adhesives, fillers and mixed with putty for stained glass windows.It's actually quite good for your health; calcium carbonate can also be used as a calcium supplement, to help build strong bones and teeth or as an antacid.

Why does meat change colour when it's cooked?
Its down to a chemical called myoglobin. Myoglobin is a bit like haemoglobin, the red coloured stuff that ferries iron around in the bloodstream, except myoglobin is locked up in muscle (and meat is muscle). Red meat contains a lot more myoglobin than white meat, as the muscles that tend to be red are the ones most active in an animal. The legs of a standing animal will be redder, and have more myoglobin, as the muscle has been tuned up for long term activity.Muscles that aren't used as often, fast-twitch muscles, tend to have low blood supply, little myoglobin, and therefore little colour (white meat). Chicken breast and wings don't get a huge amount of use (as chickens don't fly), and so they are white muscle.With fish, most of our salmon has a red colour because we tend to buy farmed salmon, and to keep the meat looking a healthy colour the fish are fed astaxanthin. They would get this in the wild environment from yeast and from algae, it's an antioxidant similar to the chemical which makes carrots orange. Shrimps eat the algae, salmon eat the shrimps and the colour passes through the food chain.

31:35 - Laki - The Biggest Atmospheric Pollution Event in History
Laki - The Biggest Atmospheric Pollution Event in History
with Dr John Grattan, University of Wales, Aberystwyth
If you think our air is polluted today, spare a thought for those living in Iceland in 1783, when an eruption in the Laki fissure caused "the biggest atmospheric pollution event in history". We spoke to John Grattan from the University of Wales, Aberystwyth. Chris - So if this volcano were industry, the government would have shut it down on health and safety grounds?John - Absolutely, it emitted about 17 times more sulphur than all of Europe today put together.Chris - So tell us about the events that led up to this eruption, how it got documented and what the consequences were.John - Well it all began in early June 1783 in Iceland, which was a very remote place at that time. A very astute pastor called John Steingrimsson started to notice a dark cloud building to the north, and that was followed by what he describes as iron filings falling to the earth and burying the plants. That was quickly followed by the smell of sulphur, and within a day or two the animals started to die, then people started to die. Within a few days, the livestock had gone. By the end of the event three quarters of Iceland's livestock (sheep, cattle, horses) had all died, along with about a quarter of Iceland's population. It was bad in Iceland.Chris - So what was the reason that those people and their animals succumbed?John - Well, there are several causes. The first one, as far as the animals are concerned, is the eruption threw out fluorine which landed on the vegetation which was eaten by the animals. That released the fluorine into their bodies, poisoning them. Essentially, their stomachs became blocked up with ash, they couldn't ruminate and the fluorine triggered uncontrollable bone growth. Sheep's teeth grew uncontrollably and they developed 'fangs' that restricted their ability to eat. Their joints developed growths which meant they couldn't walk, it was really an apocalyptic vision. The people in Iceland at the time saw it as the end of the world; as God's judgement on a sinful nation.Chris - Is it a one off, or have there been lots of examples of volcanoes having this kind of catastrophic effect before?John - I think there was an eruption on a slightly bigger scale in the 10th century, an eruption called Eldgjá which was even bigger. Probably, there's an eruption like this in Iceland, on this scale, every 500 years or so. Small eruptions in Iceland still do release fluorine and they do kill livestock, as recently as 2000 the eruption of Hekla killed livestock in Iceland. It's not just the impact in Iceland that is the problem. The Laki fissure released hundreds of millions of tonnes of sulphur into the atmosphere, and it was transported around the world. A lot of it was transported to Europe, where it also had terrible impact.Chris - So if you add up how much comes out of one volcano, how does it compare with mankind's activities? I've got an email here from Eric Taylor in the USA. He wants to know how volcanoes compare to cars, houses, or industry in terms of their emissions.John - You've put me on the spot there and that figure has slipped out of my head! But I can tell you that mount Etna, in a typical year, emits as much sulphur as all of France's industry, so it is a significant contribution. You have to remember that the planet's ecosystem has evolved with volcanoes doing this, and the problem with mans' emissions is that they are extra to this, and they often have concentrated local impacts, in cities or forests which aren't expecting to receive a contribution of sulphur dioxide. Where a volcano is emitting sulphur, the ecologies around it have grown an adapted to tolerate that.Chris - If you look further afield than just the local environment though, as you said these gases get spread right the way around the world, what can we say happens to the Earth's atmosphere, the environment and climate as a consequence of volcanoes?John - Typically, from a low intensity eruption the gases get emitted into the troposphere, they're diluted by atmospheric processes, they're rained out as very dilute acid rain and there's really no significant impact from them. Where we have large scale eruptions (which are less common, at one in five hundred years for instance) then we're dealing with hundreds of millions of tonnes of sulphur being emitted in a relatively short time. These then can be concentrated by atmospheric processes and can have a really severe impact. In 1783, when the Laki fissure eruption was taking place, a sulphurous dry fog formed all over Europe, and people were describing how their crops were being killed, leaves were falling off trees, people were complaining of the smell of sulphur, coughing and people were dying in great numbers. We think that in the summer of 1783 something like 20,000 extra people died in England alone, as a result of the environmental impact of this [eruption].We've got two things going on, the normal background typical year on year eruptive activity and these once in 500 years events which do significantly stress the environment.Chris - We've had similar things with the "London Smogs" in the 1950s though, we know that atmospheric pollution is bad for you.John - We do, and that's certainly what was the case in the 1950s. When I was beginning to investigate the Laki fissure I turned to these smog events to try and get an understanding of what may happen when you get significant concentrations of sulphur on the ground. Certainly, when you're dealing with an event like the Laki fissure, where it erupted for 8 months - people don't always realise this, they have this idea that eruptions last a few spectacular days, then it dies down. Throughout that 8-month eruption it's throwing material into the atmosphere and so there's a long-term environmental stress from something like this. In our world today if something like this were to happen we can imagine the scenario where we have atmospheric pollution in London, or Birmingham or any significant conurbation and then one morning, courtesy of an Icelandic volcano another 2 million tonnes of sulphur are delivered. That's going to push the environmental thresholds significantly, and then I think we can expect to see the significant health impacts and mortality crises.Chris - So you could actually say, because this thing blows up in Iceland you would be able to trace a health effect in the UK, in France, perhaps even further afield in Russia, China, Japan even?John - Absolutely. We've traced this, day by day, from the moment the eruption began all the way through to 1784 when the eruption ended. We've got a day by day history of the eruption and people's health, and we can see the arrival of the smog, the destruction of crops and plants and then the people dying. People begin to die in great numbers in August, September and October 1783. The people writing at the time were amazed by this, the sulphurous smog that meant you couldn't see across the valley, and they were clearly linking the arrival of the smog with deaths. There were some French priests who wrote "when the fog arrived, a third of the men of my parish were swept to their tombs". People were really scared, there's one letter which tells of the people of one parish being so afraid of this smog that they thought the end of the world was here, the gates of Hell had opened and the Devil was walking the Earth. They dragged the priest out of his house, made him put on his vestments and perform a service of exorcism on it.We've got all of this happening in the 18th century where there is relatively little industrial pollution. If an event like this were to happen now or in the future when we have significant industrial pollution and pollution in cities because of cars and industry, we're dealing with an event where the thresholds for human health impacts are much closer.
Chris - So if this volcano were industry, the government would have shut it down on health and safety grounds?John - Absolutely, it emitted about 17 times more sulphur than all of Europe today put together.Chris - So tell us about the events that led up to this eruption, how it got documented and what the consequences were.John - Well it all began in early June 1783 in Iceland, which was a very remote place at that time. A very astute pastor called John Steingrimsson started to notice a dark cloud building to the north, and that was followed by what he describes as iron filings falling to the earth and burying the plants. That was quickly followed by the smell of sulphur, and within a day or two the animals started to die, then people started to die. Within a few days, the livestock had gone. By the end of the event three quarters of Iceland's livestock (sheep, cattle, horses) had all died, along with about a quarter of Iceland's population. It was bad in Iceland.Chris - So what was the reason that those people and their animals succumbed?John - Well, there are several causes. The first one, as far as the animals are concerned, is the eruption threw out fluorine which landed on the vegetation which was eaten by the animals. That released the fluorine into their bodies, poisoning them. Essentially, their stomachs became blocked up with ash, they couldn't ruminate and the fluorine triggered uncontrollable bone growth. Sheep's teeth grew uncontrollably and they developed 'fangs' that restricted their ability to eat. Their joints developed growths which meant they couldn't walk, it was really an apocalyptic vision. The people in Iceland at the time saw it as the end of the world; as God's judgement on a sinful nation.Chris - Is it a one off, or have there been lots of examples of volcanoes having this kind of catastrophic effect before?John - I think there was an eruption on a slightly bigger scale in the 10th century, an eruption called Eldgjá which was even bigger. Probably, there's an eruption like this in Iceland, on this scale, every 500 years or so. Small eruptions in Iceland still do release fluorine and they do kill livestock, as recently as 2000 the eruption of Hekla killed livestock in Iceland. It's not just the impact in Iceland that is the problem. The Laki fissure released hundreds of millions of tonnes of sulphur into the atmosphere, and it was transported around the world. A lot of it was transported to Europe, where it also had terrible impact.Chris - So if you add up how much comes out of one volcano, how does it compare with mankind's activities? I've got an email here from Eric Taylor in the USA. He wants to know how volcanoes compare to cars, houses, or industry in terms of their emissions.John - You've put me on the spot there and that figure has slipped out of my head! But I can tell you that mount Etna, in a typical year, emits as much sulphur as all of France's industry, so it is a significant contribution. You have to remember that the planet's ecosystem has evolved with volcanoes doing this, and the problem with mans' emissions is that they are extra to this, and they often have concentrated local impacts, in cities or forests which aren't expecting to receive a contribution of sulphur dioxide. Where a volcano is emitting sulphur, the ecologies around it have grown an adapted to tolerate that.Chris - If you look further afield than just the local environment though, as you said these gases get spread right the way around the world, what can we say happens to the Earth's atmosphere, the environment and climate as a consequence of volcanoes?John - Typically, from a low intensity eruption the gases get emitted into the troposphere, they're diluted by atmospheric processes, they're rained out as very dilute acid rain and there's really no significant impact from them. Where we have large scale eruptions (which are less common, at one in five hundred years for instance) then we're dealing with hundreds of millions of tonnes of sulphur being emitted in a relatively short time. These then can be concentrated by atmospheric processes and can have a really severe impact. In 1783, when the Laki fissure eruption was taking place, a sulphurous dry fog formed all over Europe, and people were describing how their crops were being killed, leaves were falling off trees, people were complaining of the smell of sulphur, coughing and people were dying in great numbers. We think that in the summer of 1783 something like 20,000 extra people died in England alone, as a result of the environmental impact of this [eruption].We've got two things going on, the normal background typical year on year eruptive activity and these once in 500 years events which do significantly stress the environment.Chris - We've had similar things with the "London Smogs" in the 1950s though, we know that atmospheric pollution is bad for you.John - We do, and that's certainly what was the case in the 1950s. When I was beginning to investigate the Laki fissure I turned to these smog events to try and get an understanding of what may happen when you get significant concentrations of sulphur on the ground. Certainly, when you're dealing with an event like the Laki fissure, where it erupted for 8 months - people don't always realise this, they have this idea that eruptions last a few spectacular days, then it dies down. Throughout that 8-month eruption it's throwing material into the atmosphere and so there's a long-term environmental stress from something like this. In our world today if something like this were to happen we can imagine the scenario where we have atmospheric pollution in London, or Birmingham or any significant conurbation and then one morning, courtesy of an Icelandic volcano another 2 million tonnes of sulphur are delivered. That's going to push the environmental thresholds significantly, and then I think we can expect to see the significant health impacts and mortality crises.Chris - So you could actually say, because this thing blows up in Iceland you would be able to trace a health effect in the UK, in France, perhaps even further afield in Russia, China, Japan even?John - Absolutely. We've traced this, day by day, from the moment the eruption began all the way through to 1784 when the eruption ended. We've got a day by day history of the eruption and people's health, and we can see the arrival of the smog, the destruction of crops and plants and then the people dying. People begin to die in great numbers in August, September and October 1783. The people writing at the time were amazed by this, the sulphurous smog that meant you couldn't see across the valley, and they were clearly linking the arrival of the smog with deaths. There were some French priests who wrote "when the fog arrived, a third of the men of my parish were swept to their tombs". People were really scared, there's one letter which tells of the people of one parish being so afraid of this smog that they thought the end of the world was here, the gates of Hell had opened and the Devil was walking the Earth. They dragged the priest out of his house, made him put on his vestments and perform a service of exorcism on it.We've got all of this happening in the 18th century where there is relatively little industrial pollution. If an event like this were to happen now or in the future when we have significant industrial pollution and pollution in cities because of cars and industry, we're dealing with an event where the thresholds for human health impacts are much closer.

40:20 - Water as a Greenhouse Gas
Water as a Greenhouse Gas
with Professor Rod Jones & Alex Shillings, University of Cambridge
Climate change and the human contribution to the greenhouse effect has been a hot topic recently and people are gradually becoming more aware of the dangers of green house gasses.
But not many of us suspect the old H2O, to be contributing to the problem... to find out more; Azi Khatiri went to the department of Chemistry at the University of Cambridge to speak to professor Rod Jones.
Rod - Just about everybody knows that CO2 is the most important green house forcing gas, what they don't know is that water vapour in the atmosphere is about twice as effective as CO2 as a greenhouse warming gas. CO2 is put into the atmosphere by both natural and human activities. Water on the other hand evaporates from the surface and its atmospheric concentration is simply determined by the temperature of the atmosphere. So as we put more CO2 and other greenhouse gases into the atmosphere, we force the temperature to rise and therefore the atmosphere can hold more water. The additional water leads to additional warming which is an amplification factor or a feedback effect on the original forcing.
 Azi - So the more CO2 and other greenhouse gases we put in the atmosphere the warmer the atmosphere gets and the more water we evaporate, which then goes back into the atmosphere and warms it all up again?
Azi - So the more CO2 and other greenhouse gases we put in the atmosphere the warmer the atmosphere gets and the more water we evaporate, which then goes back into the atmosphere and warms it all up again?
Rod - Warms it further, yes.
Azi - What happens as radiation from the sun comes into the atmosphere and how does it interact with the water molecules?
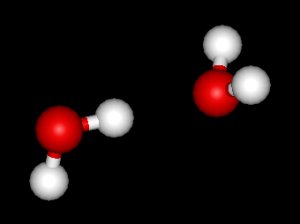
Rod - Basically, the sun's radiation warms the surface and the Earth's surface then emits infrared radiation which is at a longer wavelength than the visible light that we can see. It's that long wavelength radiation which is trapped by the water molecules in the same way that it's trapped by CO2 and other greenhouse forcing gases. The work that we are doing here is looking at part of the water vapour problem. Water vapour is a very interesting molecule in that it doesn't just absorb as an individual molecule but it can cluster together; ultimately, it can cluster together to form clouds which have a huge impact on the radiative properties of the atmosphere. We are looking at the first stages of that where two water vapour molecules come together to form a dimer.
There are two points about the dimer; one is that the absorption features are very different from the absorption features of the water molecule on its own. The second point is that as water increases in the atmosphere the concentration of the water dimer is expected to increase proportionally with the concentration of water vapour squared.
Azi - How does the water dimer differ to the water monomer, the single water molecule, in its absorption effects?
Rod - The absorption of the dimer has the potential of being stronger per molecule than of the monomer. So the purpose of our study is to measure the absorption properties of the water vapour dimer, so that we can ultimately put those into climate models which are used to predict future changes.
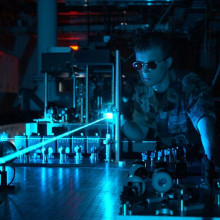
The work we do is in a laser laboratory where one of my PhD students, Alex Shillings has been working for a couple of years... we're just going to have to go through the laser interlock door... hopefully we can find Alex.
Azi - Hello Alex!
Alex - Hello, I'm Alex Shillings, and I'm a PhD student working on the Broadband Cavity Ring Down Spectroscopy experiment, and we are looking for the water dimer absorption.
This grey box you see here, this is a YAG laser producing green laser light. This green light is then injected into the blue box you see here, which is a dye laser. So we put green light in and we get red laser light out.
Azi - So what happens then to the light that comes out of the dye laser?
Alex - It comes around those turning mirrors and is injected into the cavity. Once inside the light bounces around many, many times between the mirrors; we can get an effective path of the light of 30 or 40 km even though the dimensions of the experiment is only about 2m long.
Azi - That's effectively re-producing what happens to the light in the atmosphere!
Alex - Absolutely right. That's one of the beauties of why this experiment is so appropriate for measuring species that could be important for absorbing radiation in the atmosphere, since we get paths that are commensurate with or even exceed paths of sunlight through the atmosphere.
 Azi - So you put whatever you want to measure in the tube and in this case it's water vapour isn't it?
Azi - So you put whatever you want to measure in the tube and in this case it's water vapour isn't it?
Alex - Yes, that's correct. The actual thing that we measure is the time dependence of the light leaking out of this cavity, that's basically how quickly the light decays away. We actually measure the difference between a cavity with water vapour in, or an empty cavity filled with just air, and the difference between the two loss rates gives us a direct measure of how much radiation whatever is in the cavity is absorbing.
Azi - So effectively you compare the cavity with and without the species that you want to measure and the difference between with and without, will tell you how much your species has absorbed?
Alex - That's correct. The water dimer is in fact two water monomer molecules that are stuck together by a hydrogen bond. This alters the bond between the hydrogen and the oxygen in one of the water molecules, and because this bond is altered, this leads to a new absorption feature. This is the dimer absorption feature we are looking for.
Azi - It's very interesting that you can pick up specific vibrations from the water dimer, which as Rod was telling me is very important in the greenhouse effect. Are you working in collaboration with atmospheric modellers? For example to put your findings into ways of understanding the greenhouse effect?
Alex - It has been a bit of embarrassment for climate science that since the greenhouse effect was discovered there has been a disagreement between the amount of radiation we measure the atmosphere absorbing; and the amount of radiation we predict the atmosphere should absorb from our knowledge of the composition of the atmosphere. Hopefully our measurements should be able to bridge that gap to some extent, and once we have a better understanding of the atmospheric system, we will be better equipped to deal with the climate change problems that we are almost bound to be facing in the next century.
Is the amount of water in the world increasing as we burn fuel?
Yes, but as the temperature of the atmosphere increases, it can hold more water vapour. This means that there will be more gaseous water in the atmosphere, but not necessarily more liquid water on the ground.










Comments
I want to know if rain water
I want to know if rain water from the gutters can be used and good for in door plants
Yes, it's absolutely fine.
Yes, it's absolutely fine. And, in fact, if you have alkaline tap-water in your area and you are trying to grow acid-loving plants, rainwater collected from gutters is the preferred watering choice because it has a lower pH, which will keep your plants happier.
Add a comment