This week, we're quenching our thirst for knowledge with the science of Beer and Brewing!& We learn about how beer is made, why nitrogen is vital for the perfect pint of Guinness and why professors of brewing think they have the best job in the world.& We also delve into the natural history of beer, to discuss the age old argument, what came first, the beer or the bread?& We look into the future of beer, finding out how the chemistry of carbon dioxide could provide a purer pint.& Also, a spicy way to specifically kill pain, saving slight with plastic corneas, and the hot, smelly sex lives of ancient plants.& Plus, in Kitchen Science, Ben goes for the hard stuff by learning about distillation and the science of scotch.&
In this episode

Age no impediment to hot sex
Scientists in the US and Australia have shown that age is not a barrier to hot sex, at least when it comes to one of the world's oldest species of tree. Reporting their findings in the journal Science, University of Utah researcher Irene Terry, together with Gimme Walter, Chris Moore and Craig Hull from the University of Queensland, have solved a long standing puzzle about the reproduction of cycads, primitive plants dubbed "living fossils" that date back almost 300 million years. 
Like us the plants come in male or female forms and both produce cones similar to those seen on modern fir trees. Pollen from the male cones must make its way to the female cones to fertilise them, and previously scientists thought that the wind was responsible, but subsequent studies showed that the cone scales were packed too tightly for pollen to enter efficiently. Instead it turns out that the cycads rely on thrips, tiny insects that feast on pollen, to get the job done.
But how do you pursuade pollen-covered insects to vacate a male cone and head for the female counterpart? The answer is ingenious. The cycad cones lure thrips by secreting low concentrations of volatile chemicals, including one called beta-myrcene, that the insects find attractive. The male cones produce more of the chemical than the female cones and so tend to attract more insects initially. But around midday the cones abruptly warm up, sometimes by as much as 12 degrees celsius, and remain hot until mid-afternoon, a feat they achieve by burning up sugars, starches and fats stockpiled within the cone. The surge in temperature triggers a huge million-fold increase in the production of the volatile chemicals, chiefly in the male cones. "It's takes your breath away. It's a harsh, overwhelming odour like nothing you've ever smelled before," says Terry. Just as it repels humans, the smell also overwhelms the thrips, that desert the male cones in droves, still covered in the pollen-vestiges of their dinner.
But then later, as the day goes on and the cones cool again, the odour concentration drops and the insects find it attractive again and return. But they also visit some of the female cones, mistaking them for males, and in the process deposit some of the pollen they picked up earlier. The cycle repeats itself the next day "until the males wear out and the females are happily pollinated," says Terry. So 300 million years old they might be, but they're not so unlike humans after all it seems!
Irene Terry came on the show to talk about this story - you can read the interview
here!

Researchers hit pain where it hurts
The ability to selectively switch off pain whilst leaving other nerve pathways unaffected has been a holy grail of anaesthesia for decades. But now researchers in the US have cracked the problem, with the help of a redundant anaesthetic agent and the juice of a chilli.
 Writing in this week's Nature, Alexander Binshtok and his colleagues from Harvard Medical School have found that the combination of capsaicin, the chemical that makes chillis hot, together with a local anaesthetic derivative called QX-314 that doesn't normally penetrate nerve cells, can render rats insensitive only to pain. The team injected the spicy anaesthetic mixture into the animals' hind limbs, adjacent to their sciatic nerves. The animals continued to walk around normally, but showed a dramatically reduced sensitivity to painful and thermal stimuli. Neither agent injected on its own reproduced the effect, so what was the basis of this pain-killing combination?
Writing in this week's Nature, Alexander Binshtok and his colleagues from Harvard Medical School have found that the combination of capsaicin, the chemical that makes chillis hot, together with a local anaesthetic derivative called QX-314 that doesn't normally penetrate nerve cells, can render rats insensitive only to pain. The team injected the spicy anaesthetic mixture into the animals' hind limbs, adjacent to their sciatic nerves. The animals continued to walk around normally, but showed a dramatically reduced sensitivity to painful and thermal stimuli. Neither agent injected on its own reproduced the effect, so what was the basis of this pain-killing combination?
It turns out that QX-314 does not normally work as a local anaesthetic because the molecule has a permanent positive charge. This prevents it from penetrating the oily membranes of nerve cells, which it needs to do in order to block pores in the membrane that are essential for nerve excitation, and this can only occur from inside the cell. But adding capsaicin provides the anaesthetic agent with an alternative route into the cell by opening a channel in the membrane called TRPV1. So when the capsaicin prises open the channel, the QX-314 slips inside. And because TRPV1 is only found on nerve cells that convey pain signals, only pain-bearing fibres are inactivated. The team are confident that the discovery will make a major impact on surgical and post-op anaesthesia.
"Eventually the method could completely transform analgesia, allowing patients to remain fully alert without experiencing pain or paralysis," says co-discoverer Professor Clifford Woolf.
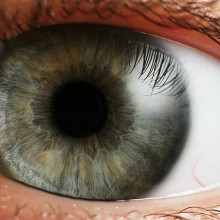
Artificial cornea could save sight
The cornea is the transparent layer that covers the front of the eyeball and protects it. But the cornea can become damaged, either through inherited disease or corrosion, and this can lead to blindness. One solution is to carry out a cornea transplant, where the damaged tissue is removed and replaced with a donated one. But there is a huge waiting list for this process, with 40,000 people in Europe on the waiting list every year.
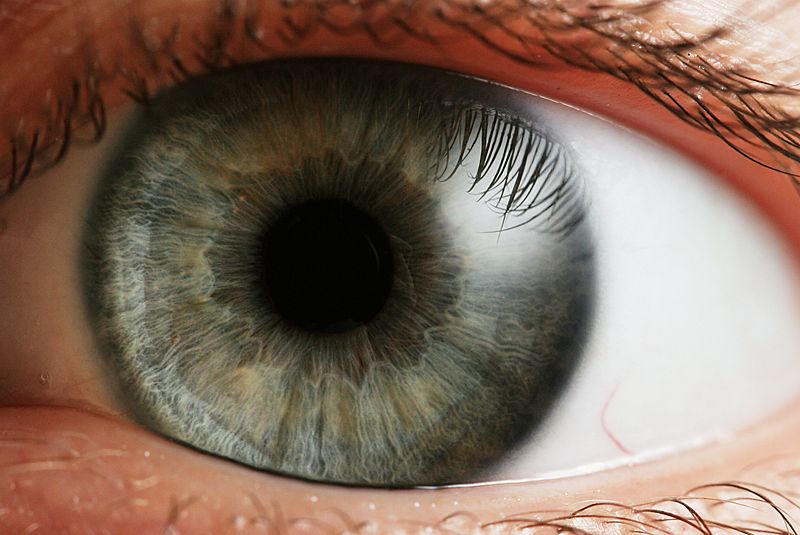 Now researchers at the Fraunhofer Institute for Applied Polymer Research IAP in Potsdam and the Department of Ophthalmology at the University Hospital of Regensburg may have found a solution in the form of an artificial cornea. Previous attempts to do this haven't been very successful.
Now researchers at the Fraunhofer Institute for Applied Polymer Research IAP in Potsdam and the Department of Ophthalmology at the University Hospital of Regensburg may have found a solution in the form of an artificial cornea. Previous attempts to do this haven't been very successful.
These new corneas are based on a polymer that prevents cells growing into it, which may cloud the vision. The artificial corneas also have a special protein border that helps them to attach to the cells in the eyeball, and have been designed to survive the high temperatures need to sterilise them before implantation.
At the moment, the scientists have tested the artificial corneas in rabbits, with promising results. If things continue to go well, they hope to test them in humans some time in 2008.

05:56 - Researchers paint a picture of volcanology
Researchers paint a picture of volcanology
Scientists have resorted to an unusual source of evidence to trace the history of volcanic eruptions on Earth and their effect on the climate - by studying paintings.
The effects of historical eruptions on the composition of the atmosphere are difficult to estimate owing to a lack of measurements or samples surviving from those times.
Now, Christos Zerefos, from the Academy of Athens in Greece, and his colleagues have come up with a proxy measure that could be used to shed light on the problem - old masterpieces.
They analysed the sunsets immortalised in hundreds of paintings from the last 500 years, including works by Turner, Klimt and Degas.
Using digital images of the paintings, the team calculated the ratios of red to green colours and found that artists used much more red after major volcanic eruptions, such as the explosions at Krakatoa in 1883 and 1680.
Next, using the red-green ratios from the paintings, the team were able to calculate how much light was being interrupted by volcanic ash particles in the air.
Their results tally well with what we know of the composition of the atmosphere at these times, suggesting that this could be an effective way to historically reconstruct the stratosphere.

07:35 - Pass me the protein stapler…
Pass me the protein stapler…
Last weekend Dr Kat was at the National Cancer Research Institute Conference in Birmingham, finding out about the latest advances in cancer science, and hobnobbing with some of the top researchers in the world. One of the most interesting talks was from Professor Greg Verdine, from Harvard University, who highlighted an important "elephant in the room" when it comes to cancer research.
Nowadays, we have huge amounts of genetic and biological information about cancer, and scientists think that these could be targets for future cancer drugs. But around 80% of these are "undruggable" - they simply can't be hit with the types of drugs we currently have. At the moment, we have small molecules. These include small chemical molecules which are probably only good at hitting about 10% of these targets, and biological molecules such as antibodies like Herceptin which can hit a further 10%.
So Verdine and his team are working on an entirely new type of drug - called synthetic biologicals, or stapled peptides. These are short stretches of protein designed to fit into the surface of crucial molecules involved in cancer. Usually, such short peptides would wiggle and flap about all over the place, and not fit effectively into their target. But Verdine has developed a way of "stapling" the two ends of the peptide together, using chemical bonds. This fixes the shape of the peptide, meaning it can only wriggle in a limited way, so it fits into its target.
And the researchers have taken it a step further, designing peptides with multiple staples - which they call stitched peptides - that are even more stable. In fact, they can stand temperatures of up to 90 degrees C, and resist the powerful enzymes found in the stomach. So this would make them good candidates for drugs to be given in a tablet form, which is ideal for a cancer drug.
So far, the researchers have been testing their stapled peptides in cancer cells grown in the lab, as well as animal models, and hope to go into clinical trials in humans in the near future.

10:00 - Hot, Smelly Sex - In Primative Plants, that is.
Hot, Smelly Sex - In Primative Plants, that is.
with Irene Terry, Univeristy of Utah
Chris - Now also in the news this week is a story about hot, smelly sex. Well, not in humans but in primitive plants. Now Irene Terry is at the University of Utah and she's found that there is a special adaptation in cycad plants that forces insects to move from a male plant to a female one during pollination. They do it by getting very hot and then releasing a very powerful, even toxic smell. She joins us now from Utah. Hello Irene.
Irene - Hello!
Chris - Thank you for joining us on the Naked Scientists. So tell us about these plants, what actually are they?
Irene - They're very primitive plants. Their lineage goes back well before the dinosaurs, called the Permian. That's when the fossils have been found. And so they made their heyday during the Mesozoic during the age of the dinosaurs. It's also gone through a lot of bottlenecks, a lot of other extinctions between the cretaceous and the modern period.
Chris - So what was the big unknown with this plant, then?
 Irene - For a while we thought these plants were wind pollinated but many researchers in the 1980s discovered there were beetles that were associated with the pollination. On sabbatical in 1999 I worked with a taxonomist who used to be the head of the British Museum actually, living in Canberra now, in Australia. I worked with him on some small insects that he found on male cycad cones. He had seen them go to female cones but he hadn't done definitive studies to show they were actually pollinating.
Irene - For a while we thought these plants were wind pollinated but many researchers in the 1980s discovered there were beetles that were associated with the pollination. On sabbatical in 1999 I worked with a taxonomist who used to be the head of the British Museum actually, living in Canberra now, in Australia. I worked with him on some small insects that he found on male cycad cones. He had seen them go to female cones but he hadn't done definitive studies to show they were actually pollinating.
Chris - So why is this such a big question? It stands to reason that if you've got a cone which attracts these beetles or these insects, why should there be a problem of them migrating between the male and female parts of the plant?
Irene - Well because most of the insects that are associated with these live only on the male cone. They lay their eggs and the larvae develop on these male cones. The question arises, why would they want to leave this nice home where they can mate, lay eggs and complete their life cycle? The male plants have to get rid of these insects to spread their pollen to the female cone. That's where the question of, 'how did they do that?' came. With the system that I've been working with in Australia, the cones start to heat up and start producing these really strong odours. If I'm standing over one of these cones it almost makes me choke because it becomes so strong.
Chris - When you say the cones warm up, how much warmer do they get?
Irene - We've measured them up to 12 degrees Celsius above the ambient temperature.
Chris - That's a lot! This is not just the sun warming them up?
Irene - No, it's not just the sun. We've actually put some little tents over them so it isn't the sun. We've measured them that way.
Chris - So how are they doing that?
Irene - They have a process whereby they're burning a lot of the sugars and starches that they've stored within the cone to increase their metabolism. Instead of making certain cellular compounds that run the cellular functions, they're losing a lot of that energy in heat.
Chris - What effect does this have on the cone when they get hot like that?
Irene - It makes the insects actually more active so we know the heat is part of the process. In addition there's this chemistry that's taking place where they're ramping up a lot of the chemistry that becomes very noxious to them. Actually in studies where we've tried to look at the behaviour of the insects, just with the chemistry alone, it can actually kill them if they're stuck inside.
Chris - So the cones are getting very, very warm and this is causing them to produce lots of these smelly chemicals which drive the insects away...
Irene - Yes
Chris - So then what happens?
Irene - Once they get to the outside of the cone all of a sudden they begin to take off in flight and they drift around in the air for a while. After these cones heat up later in the day they begin to cool off. The odours diminish quite a bit. So the insects are attracted back to the cones. Some of those may be males and the females, as well, have the same odours.
Chris - So the male cones and the female cones look very alike so the insects come back to both inevitably?
Irene - We think it's mostly the odours they're attracted to, we think there are some visual cues but we haven't actually been able to isolate them.
Chris - And the insects are presumably still covered in pollen from the male cone previously so they go into the female cone and pollinate it?
Irene - Yes, that's exactly right. That's basically how it works.
Chris - Evolution's an amazing thing, isn't it?
Irene - It is and through natural history I think we have a lot more to learn out there: just by making observations and then doing some follow-up studies.
Chris - Irene, thank you very much.
Irene - OK, Thank you!
Chris - That's Irene Terry, she's from the University of Utah - proving that the world of plants and sex is often a very hot topic!
Could chilli be an alternative to morphine?
Well, the reason people have to use morphine is because it's a very powerful painkiller and the downside of using morphine is that it does have these addicting qualities. If we can come up with a better way to block pain without producing these addicting qualities that would be really good news. The thing is that until now, no one has managed to do that, which is why I think this story is so powerful. You can home in just on the pain and not have side effects like morphine does such as addiction.

15:47 - Kitchen Science - The Science of Scotch
Kitchen Science - The Science of Scotch
with Chris Forman, Cambridge University
Ben - Hello and welcome to this week's beer related kitchen science, this is one that you can't do at home not only because heating alcohol could be dangerous, but also because this isn't actually legal to do at home, so you could get in a lot of trouble. I've come today to the University of Cambridge's chemistry department, and I'm here with biophysicist Chris Forman.
Chris - Hello!
 Ben - Today's show is all about beer, so what is it that you're going to show us?
Ben - Today's show is all about beer, so what is it that you're going to show us?
Chris - Well actually today we're going to show you how to make whisky from a substance that's actually very similar to beer. As a model substance we're actually going to use some real beer, and we're actually going to distil it in the apparatus that we've set up here.
Ben - So you make whisky by distilling something a bit like beer?
Chris - Yeah, the whisky starts off as barley, water sugar and yeast and then you let it brew. It's the yeast in that mixture that makes the alcohol, but unfortunately the yeast can't get it beyond 15%. So if we want something with a bit more kick, we're going to have to do a bit of separation to concentrate the alcohol in the beverage.
Ben - So how do you go about concentrating the alcohol?
Chris - Well, the process is known as distillation, and it's not concentration so much as separating it out from the mixture. The way that it works is fairly straightforward: If you have a liquid, ther will always be atoms and molecules evaporating, and there will always be atoms and molecules in the gas condensing back into the liquid. But when you have a mixture of liquids, for example alcohol and water, the gas that's produced will have a mixture of alcohol and water but in different proportions - that's very important, in the gas phase there's more alcohol than there is in the water. So consequently, we can capture that gas, re-condense it and then you have a liquid which has a higher concentration of alcohol and that is the process of distillation.
Ben - So it relies on the fact that different substances in your original liquid have different boiling points, effectively, and so will be present in different proportions in the vapour that comes off the liquid?
Chris - That's pretty much on the nose, although it's important to realise that the mixture itself has a boiling point and that all components will evaporate into the gas, so the gas is never a pure form, it's always a mixture. For example, in beer, you've got water, alcohol and all the tasty stuff, but in the gas phase, what you'll have is water, alcohol and all the tasty stuff just in different proportions.
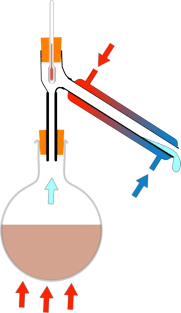 Ben - Well I can see you've got a round bottomed glass flask, and that's in a heater, and coming straight up from that is a tube with an opening in the top, into which you've put a thermometer, and an opening in the side attached to another tube that's angled down to a pear shaped flask. The tube coming out of the side has an extra glass tube around the outside of it, which has got water coming through it to make things cold. But once we've heated the beer up what actually happens to the gas?
Ben - Well I can see you've got a round bottomed glass flask, and that's in a heater, and coming straight up from that is a tube with an opening in the top, into which you've put a thermometer, and an opening in the side attached to another tube that's angled down to a pear shaped flask. The tube coming out of the side has an extra glass tube around the outside of it, which has got water coming through it to make things cold. But once we've heated the beer up what actually happens to the gas?
Chris - As the gas goes up the column above the flask, it will reach the top of the condenser and begin to condense. And it drips out of the bottom into our pear shaped flask here. Hopefully, it wont all go that way, that is to say, pear shaped, it will all work quite well.
Ben - Okay, excellent. Well I have brought along a bottle of beer that I have smuggled into the chemistry department, so shall we crack that open and get on with it?
Chris - Sure, why not? [opens bottle] Okay, so I'm pouring the beer in, and it's draining down through the column and into the flask.
Ben - So how much beer are you putting in there?
Chris - It's about 70ml.
Ben - So we're not putting too much beer in there. Is that because we don't need much? Will this get much alcohol?
Chris - It won't get that much, this is 5.6% so we should get about 3 or 4ml of alcohol out. So we've just got to let the bubbles die down, pop a thermometer in
Ben - So we've put a thermometer in above the beer, why do we need this?
Chris - Right, okay, this is very important. If you put the thermometer in the liquid, you will measure the temperature of the liquid. What matters is the temperature at which that vapour is. Measuring the temperature in the vapour phase is crucial.
Ben - Okay, so we have a tube ready to cool things down, we've got a thermometer ready to measure the temperature of the vapour, where do we go from here?
Chris - We have to start heating the beer, and that's what we'll do now!
Ben - Excellent, so we'll put the heater on. What sort of temperature do we want the beer to be at?
Chris - Well in a pure ethanol and water mixture, that will start to distill at 78 degrees Centigrade. Because the concentration of ethanol in this is much lower, the point where you get distillation will be a little bit higher.
Ben - So how long do you think it will take for our small amount of beer here to get up to the right temperature?
Chris - It wont take too long, only about 15-20 minutes.
Ben - Excellent, well we're going to leave the beer to distill, and we'll come back to you later in the show to let you know what happened. That gives me chance to go to the pub, have a pint and learn a bit more about the damage that alcohol can do to your body.
You can find the interview with Mike Allison, about the damage alcohol can do to your liver,
here.
The second part of this week's Kitchen Science, where we find the result of our distillation, is
here.

21:28 - The Secrets of Beer
The Secrets of Beer
with Professor Charlie Bamforth, University of California, Davis
There must be thousands of beers available worldwide and, of course, beer sales are very big business. But how do we actually make beer and why do we taste the tastes in beer that we taste? Well, joining Chris Smith to explain is Professor Charlie Bamforth. He is from the University of California at Davis, where he's a professor of beer and brewing. Charlie, are you really Professor of Beer and Brewing?
Charlie - Yes, it's a hard life but somebody has to do it!
Chris - I was going to say, can I have your job if you don't want it?
Charlie - No, I think I've got the best job in the world.
Chris - Haha. What does that actually involve?
Charlie - Well basically, what I do is outreach, research and teaching. I teach people all about the science and technology of brewing beer and we have a pilot brewery here and people can actually learn how to design and create their own beers. I have a very active research programme. I also have an outreach role, teaching people in California and beyond what a wonderful drink beer really is.
Chris - I suppose you must have a queue of students outside your door, wanting to join your course. When we actually talk about making beer what's actually involved in the process?
Charlie - Well, it starts in the barley fields, where you're producing really good quality malting barley. Then the malting barley has to be germinated in a very controlled way to soften it and to develop the enzymes that will break down the starch. Then the malt (the germinated barley) that is produced is dried and kilned for colour and flavour. That then goes to the brew house and is ground up and extracted with hot water. The starch degrading enzymes, the amylases, break down the starches into sugars. Then the liquid which is produced or extracted is boiled with hops to extract bitterness and aroma. This is then cooled and the pitched with yeast (Saccharomyces). And the yeast, of course, produces alcohol and some other flavours as well. Then there's a sort of cleaning-up process: some filtration, some stabilisation and it's packaged.
Chris - So why is some beer flat and other beers are quite fizzy?
Charlie - Well, yeast produces alcohol and carbon dioxide in the classic fermentation process. It doesn't produce all the CO2 that you would want in a fairly highly carbonated beer. For some beers some more CO2 is introduced but for other beers it's allowed to drift away. A classic cask ale from England is fairly low in carbonation, perhaps 1.1 volume or 1.2 volume - something like that. Some of the wheat beers in Germany are very highly carbonated, they've got three times more CO2 than liquid.
Chris - I've got an email here from Gregory Staradub. He's on the East Coast at MIT. He says, "I sometimes brew my own beer. When I open a bottle of beer that I've brewed, sometimes it behaves nicely and fizzes up all over the place but this doesn't seem to be correlated with how long the beer has spent in the bottle. Since I reuse the bottles I'm wondering, therefore, if some bacteria have got into the beer and they keep fermenting it in the bottle. Does this theory make sense and what other explanations might there be?"
Charlie - Well, there's all sorts of potential problems with brewing beer yourself. The key secret to brewing good beer is hygiene, hygiene, hygiene. It's always possible that there's residual sugar that's not been fermented properly in the fermenter, that's left behind in the bottle. Sometimes they may be leakers and the CO2 may come out of the cap. There are all sorts of possible explanations.
Chris - And Alexis Waldo says, "what makes Guinness® or stout so dark, thick and foamy and so good compared to the lighter, clear beers that you get elsewhere and places like the US?"
Charlie - Well, there are many excellent beers, some of them very light, some of them very dark. The colour of Guinness® is due to roasted cereal, roasted barley. They have a very intense heating process. The sugars and the amino acids and the grains are cooked together to give very, very dark colours. They give very roasted flavours. The foam: one of the main reasons why Guinness® foams so well is, apart from CO2 producing foam, they use nitrogen gas to give it extremely stable foams. The bubbles contain nitrogen gas and this is much more stable than CO2. Guinness® pioneered that technology.
Chris - Now, I'll let you off if you haven't got a clue about this one because, quite frankly, I was shocked! This is from Kay and she says, "My daughter recently went on a school trip and she was told that in Tudor times, if beers were poured with no head on them then they would put dead mice in the beer! Can you explain what this would achieve and why?"
Charlie - I've never heard that one! There's all sorts of crazy mythologies and truisms that people have handed down the ages. Possibly one of the things with mice is that some beer can get contaminated with Brettanomyces and Brett classically has a barnyard type, sometimes called a 'mouse urine', type of flavour. Maybe something's got lost in the telling.
Kat - Urrghh. So you're down the pub and you just put a bit of mouse wee in you pint. Anyway, we hear so many messages like alcohol is really bad for you and gives you cancer and gives you heart disease but in moderation are there actually health benefits to drinking beer?
Charlie - Yeah, there are. The red wine guys have made a bigger thing about this but it's just the same. The truth is the same for beer. One or two beers a day could stem the risk of atherosclerosis. It's been shown extensively, so it's actually good for you. Beer also is a rich source of silicon. There's a guy over there in the UK who's done a lot of work on beer countering things like osteoporosis. Beer is a significant source of several B-vitamins and antioxidants. Beer in moderation, I stress the moderation, is a good component of a diet.
Kat - Is the effect on atherosclerosis just from the alcohol content then?
Charlie - The alcohol: many people now believe the alcohol is the key ingredient in cutting down the risk of atherosclerosis. It's not some fancy antioxidant. People talk about resveratrol in red wine but you'd have to have a phenomenal number of bottles a day to get the amount needed.
Kat - Excellent, I'm absolutely gasping for a pint! Thank you very much, that's Professor Charlie Bamforth who's Professor of Beer and Brewing at UC Davis.
Chris - The job that we would all kill for!
Why does my beer sometimes explode?
We put this question to Charlie Bamforth, Professor of Beer and Brewing at the University of California at Davis. You can hear the whole interview here.Well, there's all sorts of potential problems with brewing beer yourself. The key secret to brewing good beer is hygiene, hygiene, hygiene. It's always possible that there's residual sugar that's not been fermented properly in the fermenter, that's left behind in the bottle. Sometimes they may be leakers and the CO2 may come out of the cap. There are all sorts of possible explanations.
What makes Guinness® or stout so dark?
We put this question to Charlie Bamforth, Professor of Beer and Brewing at the University of California at Davis. You can hear the whole interview here.Well, there are many excellent beers, some of them very light, some of them very dark. The colour of GuinnessÃ?,® is due to roasted cereal, roasted barley. They have a very intense heating process. The sugars and the amino acids and the grains are cooked together to give very, very dark colours. They give very roasted flavours. The foam: one of the main reasons why GuinnessÃ?,® foams so well is, apart from CO2 producing foam, they use nitrogen gas to give it extremely stable foams. The bubbles contain nitrogen gas and this is much more stable than CO2. GuinnessÃ?,® pioneered that technology.
Why did they put dead mice in the beer?
We put this question to Charlie Bamforth, Professor of Beer and Brewing at the University of California at Davis. You can hear the whole interview here.
I've never heard that one! There's all sorts of crazy mythologies and truisms that people have handed down the ages. Possibly one of the things with mice is that some beer can get contaminated with Brettanomyces and Brett classically has a barnyard type, sometimes called a 'mouse urine', type of flavour. Maybe something's got lost in the telling.

27:42 - The Natural History of Beer
The Natural History of Beer
with Dr Dave Roberts, Natural History Museum; Dr Robert Simmons, Fishbourne Roman Palace & Julian Herrington, Master brewer.
Meera - Beer is one of the world's oldest alcoholic beverages, dating back over seven thousand years and it's still one of the most popular drinks in society today. But how's it made? How was it discovered in the first place? I went to the Natural History Museum for one of their Darwin Live events, where the theme of the evening was the natural history of beer. I had chat to the event's speakers, Dr Dave Roberts: Head of Microbiology at the Natural History Museum and Dr Robert Simmons: Curator of Archaeology at Fishbourne Roman Palace. Now, although many of us are beer drinkers, not all of us know just how this beloved beverage is actually made so I asked Dave to tell me what's involved.
 Dave - Well, beers can be made from more or less any cereal-grown grain. Traditionally we use barley. It's malted, which means to germinate it which allows the starch in the grain to be turned into sugar. The sugar is then fermented with a yeast and the resulting liquor is beer. These days we add hops to make the beer bitter otherwise it's rather sweet for modern palates.
Dave - Well, beers can be made from more or less any cereal-grown grain. Traditionally we use barley. It's malted, which means to germinate it which allows the starch in the grain to be turned into sugar. The sugar is then fermented with a yeast and the resulting liquor is beer. These days we add hops to make the beer bitter otherwise it's rather sweet for modern palates.
Meera - Ok, and how has technology and the brewing process changed since that date?
Dave - Today industry is fully technological. It's using essentially large stainless steel vessels, computer controlled breweries. We have the science of brewing. We know exactly how to control it now and so you can go to a brewery, push a button and the entire process will happen under computer control. The product will be identical every time it comes out.
Meera - Now, moving onto the people that were actually drinking the beer. How far does beer go back? When's the earliest trace of people drinking beer?
Dave - The earliest definitive evidence for honest-to-goodness beer is about 3000BC. That was in Sumeria. But we have evidence from sculptures and from writing in Egypt back to about 5000BC. Cereal grains were being collected as far back as 23,000 years ago and the question then becomes, well, how do you eat cereal grains? Cereal grains themselves are very hard and so you can't just chew them because we simply don't have the teeth for it. It's much easier if you soften the grains first. If you soften them by soaking them in water then you can smash them open. At 23000 years we have no known way of cooking other than making them into a paste and putting them onto a hot stone. It's perfectly conceivable that if you were a little bit late, a little bit tardy in doing this that they would have fermented. If they were left in water, then the water would have gone fizzy and would have actually been safer to drink than stream water.
Meera - So you think it may have happened by accident?
Dave - I think it more or less must have.
Meera - How about you, Robert?
Robert - There has been an argument in the past that beer wasn't originally brewed, if you like, by accident from failed bread production. But was actually the original product and bread came later. Now it's a debate that's run on and on but it's an interesting idea and quite plausible because beer is actually more nutritious than bread would have been.
Meera - When beers, say, first started being drunk was it associated with everyday people? Was everybody drinking it or was it associated with different classes, do you think?
Robert - The way I imagine for the production and consumption of beer to have taken place, it was a big communal get-together. There were fairly small groups of people living in the English countryside and they would have brewed large quantities of this stuff possibly 100 litres, maybe 150...maybe more. It wouldn't have a long shelf life so once it's brewed it's gotta be drunk. Ethnographic examples suggest that it would be within 24 hours. Even if it's got to be drunk within 2-3 days, that's a big party! And everybody would get involved and everybody would help drink beer.
 Dave - It's also the case that in ancient Egypt we know from hieroglyphs that beer was drunk regularly with meals. With all meals, by everyone in society: from the Pharaoh right down to the smallest child. But it was also associated with at least three of the gods in ceremony. It was as Rob said, everybody seemed to drink beer because it was a major food source.
Dave - It's also the case that in ancient Egypt we know from hieroglyphs that beer was drunk regularly with meals. With all meals, by everyone in society: from the Pharaoh right down to the smallest child. But it was also associated with at least three of the gods in ceremony. It was as Rob said, everybody seemed to drink beer because it was a major food source.
Meera - So there you have it, drinking beer isn't only about the alcohol content but the nutrition as well. I guess the real question here is, what came first? The bread or the beer? Either way I'm glad it was discovered because I, for one, am a big fan of this liquid bread! Especially now that lots of pubs and bars are expanding their beer menus. But how can I tell what a truly good beer is and taste all the flavours that are meant to be in there? I spoke to Julian Herrington, a master brewer. He took me through a traditional beer tasting, to teach me how to really enjoy my pint.
Julian - First of all, you should swirl it to get the aroma off it. If you treat it like a wine, take some time over it - look at it, consider it and then swirl it to get all of those lovely citrus-y hops off it. Those orangey, lemony flavours.
Meera - So now you've got me swirling my pint, what next?
Julian - Now you have to taste it. You need to let it roll over the tongue and the tongue initially tastes the crude flavours, the sweetness, the acidity, the sourness or the bitterness. Now as it warms up on the tongue and as the carbon dioxide comes out of the beer, because there's a lot of fizz in it, that lifts the aromas. It picks up the smell, giving the deeper, more plum-y, basically fruit flavours that you can't smell until they come off the tongue as it warms up.
Meera - Mmm. That tasted good!
Julian - I'm just getting lots of raisins and fruit. Lots of fruity beers.
Meera - What type of beer is it that we're drinking then?
Julian - We're drinking a real ale or cask beer. This is one that isn't pasteurised and the yeast is allowed to sediment. It's a very natural way and it's how all beer, even beers in Europe were made before they had the fizzy, filtered beers that we have now. Beers are very complex. They have a bit more than grapes. The hops are the grapes of beers, There's the malt and all the things that we do with the malts. Crystal types of malts and lots of flavours in there. There's over 1000 brewing yeasts which can give you different flavour notes and then it depends what you do in the brewery.
Meera - Right, so that's swirl and sniff. Sip. Roll over your tongue, then look for the hang and after-flavours that come out. Got it. Next time you're at the pub, why not sample some different beers to your usual and apply these tasting techniques to see if you can find all the flavours. Who knew drinking beer could be so complicated?
How much of the flavour of beer comes from the yeast itself?
We put this question to Master Brewer Julian Herrington...
As regards fermentations of all beers yeasts can give a predominant flavour depending upon the fermentation conditions and whether one uses lighter flavours from the malt, perhaps using rice as part of the recipe, for example and how much bitterness or hop character that's used. So yeast produces fruit flavour which ranges from raisins in strong ales to a sort of peardrop and banana flavour in wheat beers. Though it can be all of the character of the beer (as in the latter example) or just part of it.

35:37 - Hop Extracts for Better Beers
Hop Extracts for Better Beers
with Dr Ray Marriott, Botanix Ltd
Kat - Now here on the Naked Scientists, we're talking about beer. God I want a drink! And we welcome to the studio Dr Ray Marriott from Botanix Limited. Hi Ray!
Ray - Hi.
Kat - So your company is working on using carbon dioxide to extract chemicals from plants? Why do you want to do this?
Ray - Well the main reason for using this technology is really to try to selectively take out the active molecules from plants so that they can be more easily used: more efficiently used, they can deliver more. In the case of hops CO2 happens to be a very good solvent and just to remove the bitterness and anti-microbial elements from hops that are actually so important in beer.
Kat - So you're basically getting the good stuff out and leaving all the cruddy stuff behind?
Ray - Yes, that's exactly right.
Kat - Tell us a bit more about how the actual process works. Looking on your website it tells us about super-critical carbon dioxide and stuff, what are you doing to these things?
Ray - Well, I think everybody thinks of carbon dioxide as a gas but CO2 can, of course be a solid as dry ice - a pure liquid, a condensed liquid. Or it can be a supercritical fluid. In liquid form and supercritical form it essentially behaves in both forms as a liquid. This can actually be passed through a column of plant material and in doing so it dissolves the materials that are soluble in that particular solvent liquid or supercritical CO2. These can be recovered using either a separator or an evaporator. And the carbon dioxide is then collected and reused and passed though the plant material. In this way, we can actually use the CO2 in what we call a 'closed loop system' to extract the active components from the plant material.
Kat - So when you talk about solvents you normally think of sort of, organic solvents and chemicals. Why don't you use something like that?
Ray - Well, using organic solvents of course is perfectly possible but they leave behind traces of the solvent within the material from which you've extracted. The beauty of CO2 is once you allow the extract to warm up, the CO2 just evaporates.
Kat - Now this all sounds very space age, but is it actually being used to make the beer in pubs?
Ray - Very much. Over 95% of the hops that are grown worldwide are processed in some way and about a third of those will be extracted using carbon dioxide and are used by many brewers as the basic ingredient for adding to the kettle.
Chris - So before we had the ability to do this with carbon dioxide, would people have just chucked into the mix and let the flavour ooze out naturally?
Ray - Yes, that's absolutely right. The whole cones of hops would have been put into the kettle. In doing that we have a relatively inefficient way of adding bitterness and aroma. This is because the components that are so important in the hops are right in the very centre of the hop cone in what is called the lupulin gland. They have to diffuse out of that and then be soluble-ised to transfer the bitterness and the aroma into the liquid. That's relatively time-consuming and an inefficient way of doing it.
Chris - And using that technique, how much of the potential flavourants would you have been able to recover from the hops: just waiting for it to ooze out that way?
Ray - About a third of the potential bitterness and aroma can be obtained from the hops.
Chris - That's not much. So you'd be throwing away potentially two-thirds of the goodness of the hops?
Ray - That's right.
Chris - And with your CO2 approach, how much can you get out that way?
Ray - We extract about 95% of the component and they can then be added directly to the kettle or later on, even post-fermentation into the beer.
Chris - And once you've got the hop extract, presumably you can see it in a bottle, what does it actually look like once the carbon dioxide has evaporated?
Ray - It's just a very viscous orangey-yellow liquid.
Chris - Does that mean you add it as-is or can you further sub-divide that and use different fractions of it to get different types of beer?
Ray - You can use the extract exactly as it comes from the CO2 extractor and many brewers do. But yes, we can take that further and then separate that into what we call functional molecules of the hop. So if there's molecules that deliver bitterness, those that deliver aroma, those that deliver the anti-microbial activity of hops: these can all be fractionated and delivered to the brewer or even to the food industry in a very useable form.
Chris - And this means that we can make beers, probably that you couldn't have made, without this technique.
Ray - Yes, it does allow brewers to add bitterness and aroma completely independently instead of putting in a hop variety with a known ration of aroma they can add a certain amount of defined bitterness and a certain amount of aroma. It allows them to create all sorts of new types of beer.
Chris - I'm very grateful to your company, Ray, for giving me such a wonderful drinking experience and I haven't forgiven you yet for turning up empty handed with no beer but just lastly, it strikes me that the same technique could be used to get all kinds of exciting molecules from plants. I'm thinking of nicotine from tobacco plants for nicotine replacement, I'm thinking of tetrahydrocannabinol from cannabis for making drug extracts that people might want to use for say, anaesthetics?
Ray - Yes it is possible. There's a wide range of plant extracts that are used from delivering just flavour and aroma right through to active pharmaceutical molecules and this, what we call a 'clean-green' technology, I think will find increasing uses in this type of area.
Chris - That's Ray Marriott, he's from Botanix limited. Thanks very much Ray. Ray's here in the studio if you'd like to ask him any questions you can email chris@thenakedscientists.com.
Kat - I'm just wondering about the possibility of beer-flavoured food - it's incredible!
41:09 - The Quality of Life Depends upon the Liver
The Quality of Life Depends upon the Liver
with Dr Mike Allison, Addenbrooke's Hospital
Ben - While we're talking about alcohol it's very important to remember that alcohol-related illness is a massive killer here in the UK and worldwide. And so to chat a bit more about that I've come to the Flying Pig pub and we're out in the beer garden I'm with Mike Allison of Addenbrooke's hospital. Hi Mike!
Mike - Hiya.
Ben - So Mike, how big a problem is alcohol-related disease in the UK?
Mike - It's a huge problem and it's an increasing problem. Our concern, particularly from the liver point of view is the trend for rapidly increasing amounts of alcohol consumption in the young with the binge drinking culture. There's evidence that this can continue with people into their early 20s moving onto consistent heavy drinking, which can result in severe liver damage.
Ben - So, when people are drinking responsibly, such as right now I'm enjoying a nice pint of bitter, what is it that actually happens to the alcohol after I've taken a swig?
Mike - It obviously goes down your gullet and into your stomach. There's a variable rate of absorption from the stomach and it enters the blood system, draining to the liver, where it is metabolised. A lot of aspects of damage that alcohol can cause is through its metabolism.
Ben - So it's the metabolites of the alcohol rather than the alcohol itself that does the damage? What does alcohol get broken down into?
Mike - It's broken down, in particular, to acetaldehyde. This is particularly toxic to cells. It can change the number of cellular constituents, affect protein, the cell wall and a number of ways in which the cell works.
Ben - So when the liver breaks alcohol down into these fairly toxic components, does that directly damage the liver?
Mike - The metabolites can damage the liver but the liver has a capacity to deal with these in a safe manner. The problem can arise, however, the system is swamped. Then the metabolites can't be degraded down a safe route. Then you do get cellular damage, which over the course of time, can lead to aggressive liver damage.
Ben - And is it the damaged liver that leads to the hangover that some of us are far too familiar with?
Mike - I think that's multi-factorial. I don't know that it's necessarily solely the liver. I think that you do get an alcohol-related gastritis and that contribute to the nausea and generally feeling awful. The neurological effects of the hangover, in terms of alcohol, will also be the dehydration one sees. Hence the advice to always drink some water before you go to bed. I think that's very sensible advice. It's multi-factorial and not solely related to the liver.
Ben - So once you've damaged your liver through the presence of these metabolites, what can this lead to?
Mike - In the long term, excessive alcohol consumption can lead to cirrhosis. You get scar tissue in the liver which is joined up and leads to architectural disruption. This leads to an increased risk of liver cancer, you can have exsanguination or bleeding from the gut. You might get fluid accumulation in the abdomen. In addition to that, what we see and what accounts for a large proportion of people turning up in hospital with liver disease is when someone has alcoholic hepatitis. That is when someone becomes jaundiced: yellow eyes, yellow skin, often with distension of the tummy - with fluid in the abdomen. This can be a very sever illness and in severe cases around 40-50% of people do not survive to get out of hospital. A worrying thing from our point of view is that we're seeing people coming in with this condition in their 20s now. It used to be people in their 40s.
Ben - So is there any way to go out, get a bit drunk, have a big night out and then not do damage to your liver?
Mike - Any night out such as that will damage the liver. It may not do so in a way that will be reflected three months later if it's a one-off. Certainly it will stress the liver. If it's done on an on-going basis or it's done nightly or regularly then you may start to see progression to what may be irreversible liver damage.
Ben - And are there ways to slow down the process of getting drunk? You hear rumours that drinking milk before you go out will line your stomach. Perhaps eating a meal before you go out?
Mike - Certainly it's the case that if you have a full stomach, if you drink with meals then that can reduce the risk of liver damage. That's not to say it's reasonable. 'If you get drunk with a decent meal that's fine for the liver' - that's not the case. But the body's tolerance of sensible amounts of alcohol with food is better than on an empty stomach.
Ben - And one other rumour that we often hear is that if you have very expensive tastes and perhaps you're celebrating if you drink some champagne, people say that fizzy alcohol goes straight to the head. Is there any truth in that?
Mike - I'm not aware of any evidence that that's the case!
Ben - Well thank you ever so much for coming to speak to me and cheers!
Mike - Cheers!
46:02 - Jacobsons organ in Humans?
Jacobsons organ in Humans?
This week's Question was answered by Peter Brennan, Department of Physiology and Pharmacology, University of Bristol...
The Jacobson's organ is part of the vomeronasal system. It senses chemical stimuli such as marks, which tell animals about the sex and individual identity of other members of their species, often called pheromones. The Jacobson's organ is found in many species but by no means, all species. The question is, whether or not there's a Jacobson's organ in humans. People have found a small in-folding in the nose of humans and claimed that to be a Jacobson's organ. The question is, whether or not it is functional?
The overwhelming weight of evidence is against this function. For instance, it has a very different appearance from the Jacobson's organ that you find in other species. There are no nerves that can be found that connect it to the brain and therefore it is unlikely that it can send a signal to the brain. Moreover, there are genes that are vital for the function of the vomeronasal organ/Jacobson's organ in other species but they don't work anymore in humans. They become redundant during human evolution. That happened at about the time when the new and old world monkeys split off from a common ancestor in Africa, many millions of years ago. So it seems very unlikely that the Jacobson's organ can have any function at all in humans.
Diana: So there is some evidence for its existence in humans, but it seems unlikely that our brains are actually receiving any information from it. It may be that our ancestor hominids had one to detect sex pheromones. It's more likely now though that you can only tell a man from a woman from their scent, because there are a lot of boys out there who smell pretty awful anyway. Otherwise it may be worth memorising a pour homme or pour femme perfume counter.

50:10 - Kitchen Science - The Science of Scotch Part 2
Kitchen Science - The Science of Scotch Part 2
with Chris Forman, Cambridge University
This is the second part of this week's Kitchen Science, you can find the first part
here.
Ben - Hello again, welcome back to kitchen science. While I've been down at the pub, [to find out about the
effect of alcohol on the liver] Chris has been keeping an eye on our distillation, and I can see that in the bottom of his pear shaped flask, he's actually collected a few milliliters of clear liquid. Chris, what's happened while I've been away?
Chris - Well you missed the exciting bubble moment, when all the beer frothed up, we've got a little pressure release valve so that wasn't a problem. What's happened is the alcohol and water mixture has come out of the beer, condensed at the top, come down the tube and collected in this flask here. We now have what should be almost pure alcohol in this glass.
Ben - So can we take this out and have a closer look at it?
Chris - We're not allowed to drink it, that would be very bad for you. It's slightly yellowy, so that means that some of the products in the beer have come through as well as the alcohol itself, which is what you would expect.
Ben - But it's no-where near the colour of the beer, so presumably the compounds that make up the colours in the beer have been left behind.
Chris - The vast majority of them have, yes indeed.
Ben - So what we have left is what could be very tasty but almost completely non-alcoholic beer.
Chris - Well there would still be some alcohol in there, but a much smaller proportion, as most of the alcohol has come out into this flask. In fact, if you kept the process going, what would happen is that different compounds would come out of the beer. If we wanted to we could put another flask on, and collect another type of substance. In the whisky distillation process, which is what we're looking at, it's very important that different stages of the distillation are siphoned off in different directions. That is, indeed, the art of the whisky distiller - to control the flow of the fluid our of the barrel and into the final drink. Incidentally, you cant call it whisky until it's been maturing in a barrel after the distillation process for three years.
Ben - So if distillation is such a useful process for separating and purifying different chemicals, other than making strong alcoholic beverages, what else do we use it for?
Chris - Well it's fantastically useful for all kinds of things, taking crude oil; distilling it into paraffin, into kerosene, into gasoline, all the different kinds of oil. Desalinating salt water to get freshwater and salt. Any sort of process where the boiling points of the liquids involved are more than about 10 degrees apart, you can use distillation to purify or identify individual compounds in a mixture of fluids.
Ben - So it's a really useful process we can use to separate out all sorts of things and get the different products out of crude oil and that sort of thing, but how long has distillation been used?
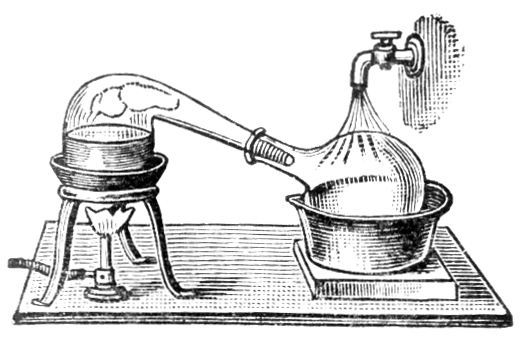 Chris - Distillation has been around for millennia. Originally it was Babylonian alchemists in Mesopotamia, that's the first known set of distillation equipment, but it was refined into it's modern form by, of all people, a Muslim Arab in about 800AD. In fact, the word alcohol itself is Arabic, and it means 'finely divided', which is very interesting. Of course, the Arabs used it to make perfumes, rather than alcohol.
Chris - Distillation has been around for millennia. Originally it was Babylonian alchemists in Mesopotamia, that's the first known set of distillation equipment, but it was refined into it's modern form by, of all people, a Muslim Arab in about 800AD. In fact, the word alcohol itself is Arabic, and it means 'finely divided', which is very interesting. Of course, the Arabs used it to make perfumes, rather than alcohol.
Ben - Okay, so I have been down to the pub, and in the time that I've been away a little bit of liquid has collected in your pear shaped flask. For all I know, that could be a bit of water that you've put in there, so is there any way that we can prove that is the alcohol distilled out of the beer?
 Chris - Well normally you would measure the specific gravity, and when they measure the specific gravity of whisky, customs and excise keep that section under lock and key, so that the brewery can't cheat and say that it's stronger than it really is. What we can do today, because it's to small to measure the specific gravity, is to set fire to it, and if it burns that proves that it's flammable, and most likely to be ethanol.
Chris - Well normally you would measure the specific gravity, and when they measure the specific gravity of whisky, customs and excise keep that section under lock and key, so that the brewery can't cheat and say that it's stronger than it really is. What we can do today, because it's to small to measure the specific gravity, is to set fire to it, and if it burns that proves that it's flammable, and most likely to be ethanol.
Ben - Brilliant. Obviously this is something we should do somewhere safe, I guess?
Chris - Of course, yeah. I'd just like to point out that we have all been wearing safety spectacles and appropriate safety equipment and we should do the burning in a fume hood.
Ben - Okay, well lets pour this liquid out into a beaker, take it to a fume hood and see if it lights...
Chris - A very very small amount.
Ben - Lets see if we can get this lit... ...Oh!
Chris - There was a flash, of blue.
Ben - Well that was very brief, but there was clearly a flash of blue flame there, that did ignite. So I guess this proves that you have distilled alcohol out of beer!
Chris - That's correct, probably what we've got here is a slightly larger amount of water than we wanted, but a lot of the alcohol came out in one go.
Ben - And that's all we have for kitchen science this week, but we will be back with more next week, so all that remains is to say thanks to Chris.
Chris - Thank you!
Ben - And goodbye...

What in WD40 kills spiders?
Chris & Kat discussed this question in the studio with Ray Marriott...
Kat: I think it's probably blocked its breathing apparatus or something like that. That's a horrible thing to do. You should let it out, let it run free!
Chris: Ray, what do you reckon?
Ray: Probably a solvent - which is actually carrying the penetrating oil - probably asphyxiated it first and then it blocked its spiracles afterwards.
Chris: Because most volatile things can act as general anaesthetics; so when people inhale lighter fluid and things, they're actually putting all that gas into their brain. It seems to cause brain cells to decrease their activity, so it could be that it anaesthetised it and then, as you say, asphyxiated it.
Kat: Maybe it died high as a kite?
Chris: You never know!
Where does industrial carbon dioxide come from?
We put this question to Ray Marriott of Botanix Ltd. You can read our entire interview with him here.
I think that's a really interesting question. There are processes such as fermentation and the production of fertilisers which actually generate quite high volumes of carbon dioxide. It's from these processes that we actually capture and purify the carbon dioxide and then use it for extraction. It's then used in multiple extraction so it's actually quite and efficient way of using it. And it's actually a neat way of capturing CO2 from the brewing and fermentation process and reusing it to extract one of the raw materials.
Is there any danger to health from drinking cider?
Kat: I would think there probably is if you get really drunk, fall over, do something stupid. Chris: Well, cider's a little bit stronger than beer, on average, isn't it? So, I would think enjoyed at the same rate as any other alcoholic beverage and within guidelines prescribed by the government and not more than 21 units per week for men or 14 units per week for women. I can't see any reason why it would be worse for you but obviously in excess it's gonna be bad. Do you think it counts as one of your five a day? Kat: I don't think it counts as a fruit and veg, no!

Why do we cry when we laugh?
I think that's because you're scrunching up your face in hysteria. It's actually squeezing out the tears from your tear ducts. It's also blocking the ducts in your nose so it's all pouring out of your eyes.
- Previous Naked Science Q&A Show
- Next Smart Materials









Comments
Add a comment