How can science save the Tasmanian Devil? New research reveals why an infectious cancer that's spreading amongst the animals isn't attacked by the immune system. Plus, the quantum basis of smell, reading a fish's thoughts and are scientists on the verge of a cure for the common cold?
In this episode

07:42 - Fossilised fish worm eggs from 270 million years ago
Fossilised fish worm eggs from 270 million years ago
A rock unearthed in Brazil is the fossilised faeces of a fish from a quarter of a billion years ago, and it contains the oldest-yet-discovered examples of  intestinal worm eggs.
intestinal worm eggs.
The 5cm by 2cm coprolite, which was examined by Federal University of Rio Grande researcher Paula Dentzien-Dias, came from the Sao Gabriel region of southern Brazil in what would have been, 270 million years ago, a freshwater lake.
The faecal fossil, which contains scales and bone fragments, also has a series whorl shapes at one end characteristic of elasmobranche fish like skate, rays and sharks.
But under the microscope, also visible were a cluster of nearly 100 unsual, oval-shaped structures about one tenth of a millimetre across.
Examined more closely they are typical of tapeworm eggs, one of which even contains a developing worm larva.
Writing in PLoS One this week, the team point out that, what they describe as an "amazing discovery", shows that parasites were around since the advent of life.

09:56 - Seeing a fish's thoughts
Seeing a fish's thoughts
A new imaging technique has allowed scientists to see thoughts occurring in real time as fish contemplate their prey.
Japanese researcher Akira Muto and colleagues, writing in current biology, genetically engineered zebra fish to produce a glowing green label called GFP in an area of the brain called the optic tectum, which is involved in decoding vision. The green glow was switched on whenever nerve cells in this part of the fish brain became active, meaning that different regions of the optic tectum quite literally "lit up" when the fish looked at different things.
What did they find?
First, they showed that the brain cells in the optic tectum responded when a spot was flashed on and off in the fish's visual field. They then moved the spot around and found that the area that lit up also moved around this region of the brain.
spot was flashed on and off in the fish's visual field. They then moved the spot around and found that the area that lit up also moved around this region of the brain.
This is known as "topographic mapping". It occurs because the brain is laid out like a map of the fish's visual world, so one part of the eye always sends signals to the same part of the brain.
They also offered the fish a "paramecium", which is a small organism that young zebra fish like to eat. When the paramecium moved around in front of the fish, nerve cells lit up in the optic tectum on the opposite side to the side the prey was on. This is because the brain is cross-wired- the left hemisphere controls the right side of the body, and vice versa.
And when the fish began its prey capture behaviour: approaching the prey and pursuing it with its eyes, one particular part of the tectum, towards the rear, always became active. The researchers suggest that this area may therefore be connected to a brain region that produces these prey capture behaviours.
This technique could also be used to look at other regions of the brain, as fish can be bred to express the fluorescent protein in other areas of interest. It is particularly useful as you can see the changes in real time, and it is relatively non-invasive, so the animals can be studied when behaving in a natural way. This allows researchers to find out more about how the brain controls movement and hunting.
Why did they use zebra fish?
Zebra fish are rather special as they are transparent when they are in the embryonic and larval stages- that is before they are fully grown. They also grow rapidly, and a fertilised egg can become a fish able of catching prey after only 4 days. This means that fish can be bred with this fluorescent protein, and you can actually watch them, under a microscope as they are very small, and see which bit of their brain glow when they see different moving or stationary stimuli!
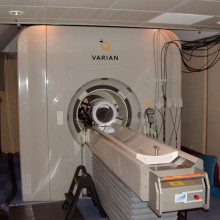
13:54 - Improving the power of MRI
Improving the power of MRI
Two papers in this week's issue of Science report on efforts to improve the resolution of nuclear magnetic resonance spectroscopy, which may 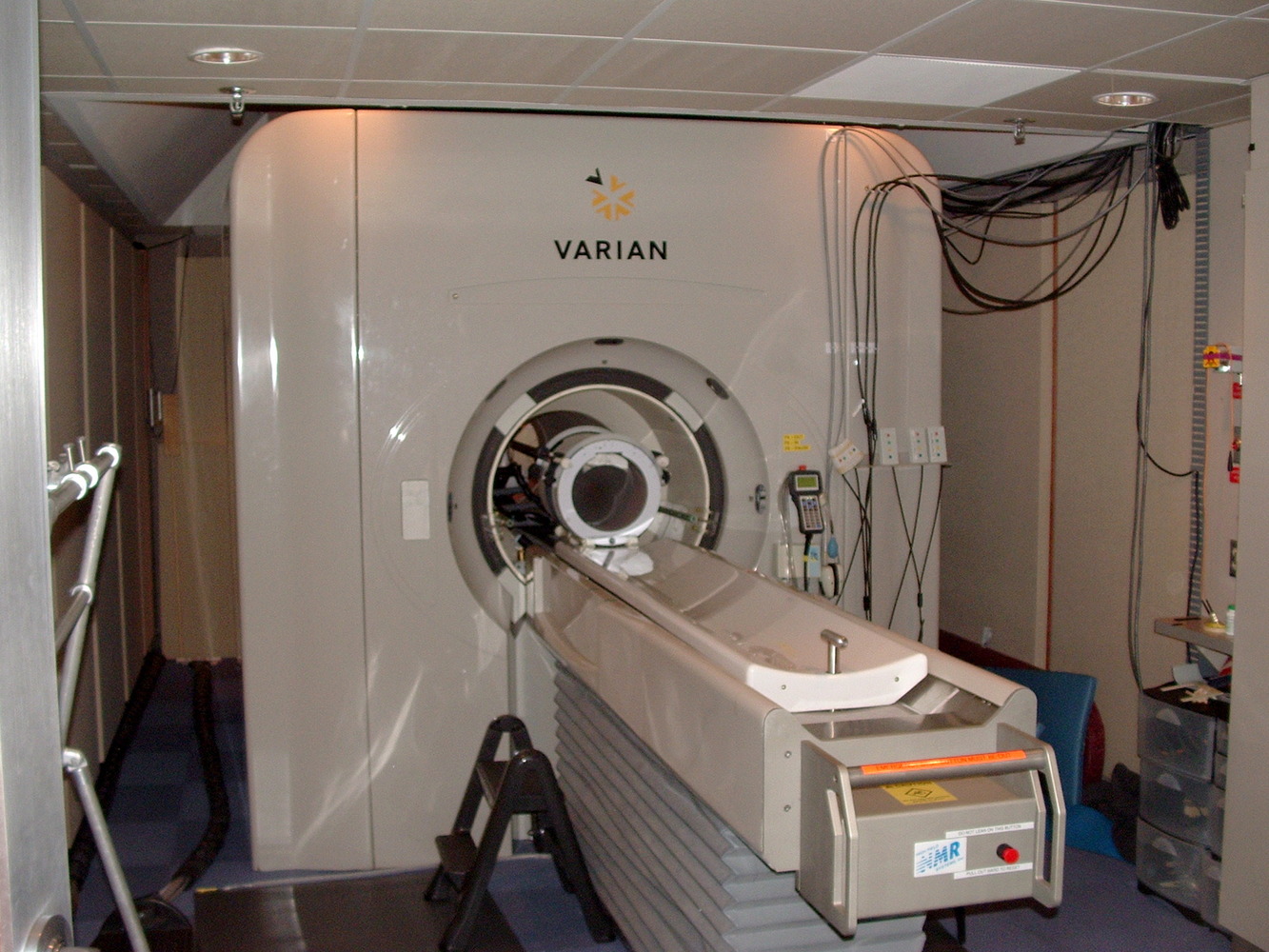 pave the way for a new, nanoscale imaging technique.
pave the way for a new, nanoscale imaging technique.
Magnetic Resonance Imaging (MRI) is a commonly-used technique in the medical sciences, because it can be used to see inside opaque objects - such as a person - non-invasively and without using ionising radiation like X-rays.
A related technique, Nuclear Magnetic Resonance (NMR) spectroscopy, is used by scientists to investigate the properties of molecules like proteins.
Both approaches rely on positive charges (protons) in the nuclei of materials being excited by bursts of radio waves.
These radio pulses alter the way the charges 'spin', causing the charges to emit their own, brief radio signals which are, in turn, detected and used to build up a picture of the molecular structure of the object - or tissue - under scrutiny.
In a hospital MRI scanner, patients lie inside a powerful magnetic field, which aligns the spins of all of the protons in the hydrogen atoms in the body. When radio signals are briefly applied, the proton spins are momentarily knocked off kilter. As they snap back into alignment with the field, the scanner picks up the signals they emit to produce a three-dimensional image.
But the signals are extremely weak, meaning that they readily merge into the background magnetic "noise", limiting the resolution of medical magnetic resonance imaging. So far, the only way to improve the resolution has been to keep everything extremely cold (just a few miliKelvin), which is a little impractical.
Now, step forward "nano-nuclear magnetic imaging", the brain child of two independent research groups, one led by John Mamin at IBM's Research Division in California and the other by Thomas Staudacher at the Max Planck Institute in Stuttgart. Both are confident that this new approach can image individual molecules measuring just a few nanometres in length, and at room temperature.
It uses a structure called a diamond-based magnetometer. This comprises a thin layer of diamond (carbon atoms) in which some of the carbons have been replaced by nitrogen atoms - this introduces into the diamond lattice "vacancies", which acts like extra electrons, making the surface very sensitive to the presence of even very tiny electromagnetic fields.
This makes it perfect for sensing the weak signals generated by the changing spins of individual nuclei, pushing the potential resolution down to a level at which it may be possible to use MRI to see inside individual living cells.
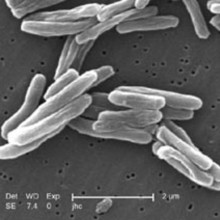
17:50 - So that's where tuberculosis hides
So that's where tuberculosis hides
In a move that will make tuberculosis (TB) easier to treat, scientists have discovered a hidden hang-out used by the bacterium to evade immune capture or destruction by antibiotics...
Writing in Science Translational Medicine, Antonia Campos-Neto, from the Forsyth Institute in Massachusetts, and his colleagues have flushed out a previously unknown hiding place where TB can lurk beyond the reach of drugs or the immune system.
Using experimental mice, the team have discovered that TB bacteria (Mycobacterium tuberculosis) can get inside the stem cells that make up the bone marrow and lurk, in an inactive state, invisible to the immune system.
Bone marrow stem cells are an ideal target because they divide, rather than die, so the TB bacteria always have a home, the cells reside in a so-called immune privileged site, where the immune system is held in check, and these cells produce a molecule called an ABCG2 efflux pump, which rapidly removes anti-TB drugs from the cells.
In tests, the team were able to show that TB could be successfully cultured many months later after mice were infected via the airborne route with TB.
Even more convincing was the finding that the bug could also be grown from bone marrow samples obtained from previously-treated human TB cases, who were otherwise regarded as cured.
The good news is that the infected stem cells that the team have identified carry a specific marker on their surfaces called CD271. So now a targeted therapy can be developed to ensure the bacteria are flushed out from these cells too.
This cannot come a moment too soon: official figures show that over 2 billion people worldwide are currently TB infected...
21:36 - Cellular Housekeeping could block HIV
Cellular Housekeeping could block HIV
with Professor Beth Levine, University of Texas Southwestern Medical Centre
Researchers have announced that they've come up with a new way to block the growth of viruses and this might even offer us a cure against the common cold.
Beth Levine from the University of Texas Southwestern Medical Centre has discovered a molecule that can activate a system called autophagy- the cells used to remove waste including any viruses, and even bacteria that are also trying to grow in the cell.
And she got the idea from HIV which actually turns off this system. So, turning it back on hinders virus growth, and what's more, the discovery might even hold the key to treating degenerative brain diseases like Huntington's disease too.
Beth - When we embarked upon this study, we were interested in trying to understand how viruses disarms the essential mechanism that cells use to defend themselves against viruses.
Chris - What is that?
Beth - That is a cellular pathway called autophagy. In very simple terms, you can view it as a cellular garbage disposal mechanism. Basically, that way of "cleaning up a trash" as we say in the US and getting rid of all the harmful or unwanted things inside the cell such as damaged organelles or bacteria, or viruses that have gotten inside the cell or miscoded proteins that can cause disease.
Chris - And so, cells can just turn this process on or increase the rate at  which it happens if they need to do deal with an accumulation or some feat of trash, as you put it?
which it happens if they need to do deal with an accumulation or some feat of trash, as you put it?
Beth - Yes, so all cells do this all the time. It is essential for cellular survival. When they're confronted by different kinds of stress they do so and viruses and intercellular bacteria have found ways to outsmart that.
Chris - Do they need to outsmart the process because if you've got a virus trying to grow in a cell, then the viruses causing the cell to accumulate various things which were actually going to turn into ultimately bits of virus and were the cell to throw those away would obviously hamper the ability of the virus to grow? So, by inhibiting this disposal system, the viruses optimising or improving the efficiency which is able to grow in a cell.
Beth - Exactly, yes.
Chris - And so, what viruses have evolved to have strategies that mean they can turn this off?
Beth - So far, the viruses that we know about that turn this off are HIV-AIDS, influenza virus, and several different herpes viruses.
Chris - So, the fact that it's so common in so many different types of virus tells us that this is obviously really important for the ability of viruses to grow efficiently in cells. And so, were you to turn the tables and find a way to turn it back on again, so the viruses can't block it, you might potentially have a whole new way to treat lots of these infections?
Beth - Exactly. It's turning out that many different viruses as well as many different bacteria have multiple different strategies to block autophagy. Even the same virus can have many proteins that block autophagy, so that I think illustrates just how important it is for their own survival and if we can prevent the virus from outsmarting the host cell and bypass the block that the virus puts, pathogy that could be a strategy for treating viral infections.
Chris - Have you managed to find a way to stop viruses from turning off this system?
Beth - Well the peptide that we recently discovered which we reported in the article that came out in Nature today is composed of 18 amino acids from the autophagy protein beclin-1, one of the essential proteins that's necessary for autophagosomes to form.
Chris - These are the structures in cells that do the breaking down?
Beth - Right and it can increase autophagy in virally infected cells, and we have shown in cell culture systems that it can inhibit the replication of HIV, and intracellular bacteria called diphtheria and to mosquito-borne viruses, chikungunya virus, and west Nile virus. And we also showed in animal studies in mice infected with chikungunya and west Nile virus that administration of this peptide could reduce levels of infectious virus in their tissue and reduce mortality.
Chris - So, you have this short little protein which when you put it onto cells does appear to have this very powerful anti-viral effect and it seems from what you're saying to actually work against a range of different viruses. So, does this mean that a.) we could find a way of doing this in pill form and that ultimately, we will be getting a cure for the common cold?
Beth - Well, I couldn't claim that we have the answer yet, but this is something that people then kind of seek out for decades or centuries, but I think that the goal would be, as you're eluding to, I think having the sequence of the peptide that we have and further understanding of its mechanism will enable collaborators in the industry to develop a small molecule that could mimic the actions of its peptide. And the goal would be to move this into human trials and see whether it could be a cure for different viruses and other diseases.
Chris - Just to finish off and the key is in what you just said 'other diseases'. There are a range of other diseases that are nothing to do with viruses, we don't necessarily think, but they do cause rubbish to build up inside cells, these neurodegenerative diseases like Huntington's. Now, if you could activate or soup-up autophagy in those cells, so that of that accumulating rubbish which we think leads to the destruction of nerve cells and causes the disease, does that mean we might also here have an answer to dealing with a whole raft of these nerve-disabling disorders?
Beth - I would like to be cautious in saying that there are a lot of steps that we need to take to first, confirm the safety of this approach in animal models. But in principle, I think there's a strong likelihood that that could work.
Chris - Beth Levine from the University of Texas Southwestern Medical Centre and that study was published this week in the journal Nature.
28:12 - Ocean Fronts - Planet Earth Online
Ocean Fronts - Planet Earth Online
with Peter Miller and Kylie Scales, Plymouth Marine Laboratory
In winter, the sea might look dull, grey and lifeless, but the creatures that live there know that some areas have far more food than others. Fish are particularly fond of ocean fronts where masses of warm and cold water meet, as these tend to contain large quantities of plankton. Planet Earth's Richard Hollingham has been to see Plymouth Marine Laboratory's Peter Miller.
Peter - In the atmosphere, you get warm and cold air meeting. It's similar in the ocean. You get a big mass of cold water meeting warm water, and along that dividing line, you get mixing processes and that mixing can keep nutrients coming to the surface and that gives rise to plankton blooms, and it keeps the plankton growing for longer. Those are the areas where fish and larger animals have learned to find better foraging opportunities.
Richard - So, you're studying these ocean fronts. What are you looking at?
 Peter - We use satellites that are orbiting the earth everyday to map out the sea surface temperatures. And over the years, I've developed algorithms to automatically pick out and combine the locations of those fronts. So even though most of the ocean's covered by clouds, we can piece together a view of where these fronts are, simplify them so that you can see one line and then can relate those positions to where the animals are.
Peter - We use satellites that are orbiting the earth everyday to map out the sea surface temperatures. And over the years, I've developed algorithms to automatically pick out and combine the locations of those fronts. So even though most of the ocean's covered by clouds, we can piece together a view of where these fronts are, simplify them so that you can see one line and then can relate those positions to where the animals are.
Richard - What sort of animals are we talking about here? You mentioned fish.
Peter - Yeah, we've been studying animals from fish, basking sharks, dolphins, sea birds, all of the top marine predators like seals, turtles. It's surprising how many animals and how many scientists we found so interested in, relating where the animals are to these productive frontal zones.
Richard - And one of the scientists doing that work is PhD student at the Plymouth Marine Laboratory, Kylie Scales who is also with us on the rocks. What animals are you looking at?
Kylie - I have been using satellite animal tracking data to look at the movements of grey headed albatrosses in the southern ocean, the northern gannet in the Celtic Sea, and loggerhead turtles in the Mauritanian upwelling region - just some of the species that potentially might be targeting that foraging effort in frontal zones.
Richard - And some of the species, there are pretty exotic places around the world, but some of the species around the coast here include some of these big fish like basking sharks.
Kylie - Yes, so the basking shark is the second largest fish in the world. They range between an average of 2 to 5 metres and we frequently see them around the British coast, particularly around hot spots in the Isle of Man and around Cornwall. They forage primarily on zooplankton. So, these areas where you get enhanced zooplankton abundance like frontal zones could be really significant features in the foraging seascape of basking sharks.
Richard - And these are really curious-looking creatures because as you say, they are massive. Their head, its enormous and even though they're massive, they're just eating zooplankton.
Kylie - That's right. Their primary foraging strategy is really just to swim along near the surface where you get enhanced zooplankton abundances with their mouths wide open, and hoover up anything that might be in their path.
Richard - And a point to this Peter is to relate these ocean fronts to conservation.
Peter - Yes. What we've been able to do is, with evidence that we're building such as Kylie's PhD, about the importance of these frontal zones to different animals, we can then start to use fronts as a proxy for increased abundance or diversity of animals like dolphins and sharks, and seabirds. For the UK effort to set up new marine protected areas, we were able to feed our data on the distribution of fronts and that's been used quite widely in the project to setup the boundaries of these protected areas, to ensure that they conserve some of these pelagic animals.
Richard - So, understanding where these are, how they move, it's quite a big deal really.
Peter - It is and now that we've done the work for the UK, we're now collaborating with American scientists to look at the open ocean because there's a lot of concern about how difficult it is to conserve the marine life out in the wide ocean. And efforts are underway to start to piece together data that can allow the most important areas of the ocean to be protected.

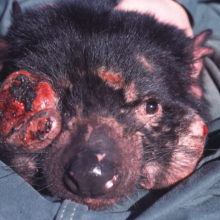
33:14 - Saving Tasmanian Devils
Saving Tasmanian Devils
with Dr Elizabeth Murchison, Wellcome Trust Sanger Institute
Tasmanian devils are in danger of extinction, and to a contagious cancer that's spread between animals by biting. It's called devil facial tumour disease.
Devil (A) with a tumour bites devil (B), introducing some of the tumour tissue in the process. The tumour then takes and devil (B), also develops the disease and will most likely die within a few months.
The disease has claimed now more than 60% of the Tasmanian devil population since it was first spotted 16 years ago. At first, no one knew what was causing it until Tasmania based genetic expert, Anne-Marie Pearse looked at the chromosomes in the cancers, and made a startling in discovery back in 2006.
Anne-Marie - Back then I was studying human cancer and I'm a bit of a  crossbreed because I started out as a zoologist and switched to looking at chromosomes in human cancer which I did for 17 years. So, when this tumour came up, I walked in and told them I thought that maybe I could help. When we looked at the chromosomes in the actual tumour itself, we found that there were very, very mixed up chromosomes, just a complete mess. But the really interesting thing was, we found that they were exactly the same in every devil. Now, when you get something as complicated as the mix-up in these chromosomes in this cancer and when you can't find any sex chromosomes in the cancers in animals of either sex, you start to think, "Hang on, we've got an infectious cell line."
crossbreed because I started out as a zoologist and switched to looking at chromosomes in human cancer which I did for 17 years. So, when this tumour came up, I walked in and told them I thought that maybe I could help. When we looked at the chromosomes in the actual tumour itself, we found that there were very, very mixed up chromosomes, just a complete mess. But the really interesting thing was, we found that they were exactly the same in every devil. Now, when you get something as complicated as the mix-up in these chromosomes in this cancer and when you can't find any sex chromosomes in the cancers in animals of either sex, you start to think, "Hang on, we've got an infectious cell line."
Chris - So, now we know the cause of the condition, scientists have been trying to work out how to stop it.
Hannah Siddle is based at the Pathology Department at Cambridge University where she's been looking at the immune aspects of the disease. For instance, why isn't the foreign tumour tissue attacked by immune cells? We'll hear from Hannah in a minute, but first, Elizabeth Murchison, also from Cambridge University, and the Sanger Institute, works on this disease, and has been looking at ways to reverse the decline in Tasmanian devil numbers. Elizabeth, tell us, for people not in the know, what actually is a Tasmanian devil?
Elizabeth - Well, most people in the UK often think that Tasmanian devils are a cartoon character that spins around and around. In fact, Tasmanian devils are the largest remaining marsupial carnivore. A marsupial is a mammal like a kangaroo that has a pouch and although most of us are familiar with marsupials like kangaroos and koalas, they also include carnivorous marsupials which eat meat, and the Tasmanian devil is actually the largest of these in the world.
Chris - How big is large?
Elizabeth - Well, it's about the size of a smallish dog. So, the males are actually about double the size of females and they weigh about, up to 13 or 14 kg.
Chris - And now, they're just confined to Tasmania, but were they once right across the Australian mainland?
Elizabeth - Yeah, there's fossil evidence that shows that Tasmanian devils used to be found all across the Australian mainland. They went extinct in the mainland of Australia about 1,000 or 500 years ago. We're not quite sure why and now, they're only confined on the island of Tasmania to the southwest mainland of Australia.
Chris - And a high proportion of them are succumbing to this disease.
Elizabeth - That's right. I mean, we don't know the exact numbers but it seems that more than 60 % of devils have disappeared as a direct consequence of these diseases spreading through their population. And in some areas on the east coast of Tasmania where the disease is best observed, more than 95% of the devils have already gone.
Chris - Are they beyond the tipping point or do you think we can save them?
Elizabeth - Well, it's really difficult to prevent this disease from continuing to spread in the wild because now, it's spread through almost all of the Tasmanian devils' habitat. So, there's a few different options in trying to protect devils in the wild. One of them is trying to prevent the disease from spreading further either through building barriers such as fences to prevent the disease spreading, or by coming up with some kind of intervention vaccine or cure to try to keep the disease under control.
Chris - Which of those is proven the most successful has got the most support at the moment?
Elizabeth - Well, one of the most important conservation efforts that's going on at the moment is translocation project which is actually taking devils from the wild in Tasmania and putting them onto an island off the coast of Tasmania called Maria Island, which previously didn't have a devil population. But has now become a haven for disease-free devils and we're hoping that devils are going to continue breeding there in the safety of isolation on the island without the disease, so that if the disease actually does wipe out the devils in the wild, they could be reintroduced from this island.
Chris - Will there be enough genetic diversity in such a small geographical area compared with the normal range they would enjoy where they're not confined to the island?
Elizabeth - Yeah, well that's a big concern and worry. Of course, when you take a small population of individuals and keep them breeding in captivity, you lose genetic diversity very quickly. And especially if you want to reintroduce this population to keep the species going, it's really important to capture that genetic diversity to keep species rigorous and healthy.
So, at the moment, we're trying to map the kinds of genetic diversity which is already present in the devil population in order to select the best devils, to maximise the genetic diversity in the captive island population.
Chris - Because originally, people did think that perhaps the reason that this tumour problem was coming along was because there was a lack of genetic diversity. Do your results bear that out or are the devils that are left in Tasmania relatively inbred, or is there still quite good diversity at the moment?
Elizabeth - They are relatively inbred. They seem to have much less genetic diversity than many other wild species in Tasmania. However, we don't think that this is the reason for the spread of the disease. Actually, this is work from Greg Woods and Alex Kreiss in Tasmania who showed that they can do skin grafts of skin between devils that even appeared to be very genetically similar to each other, and that the skin grafts, were rejected by devils even though the tumours which are also from different devils were not rejected. So, there seems to be something very special about this tumour which is preventing it from being detected by the immune system.
39:51 - How Tumours Evade the Immune system
How Tumours Evade the Immune system
with Hannah Siddle, Cambridge University
Devil Facial Tumour Disease, the cancer spreading though Tasmanian Devil populations, is able to evade the immune system. New epigenetic studies show how...
Chris - What is special about this tumour tissue that when injected into a devil, by a devil (or perhaps even by a human if we're to do that experiment) means that there isn't an immune attack against it because we're out to take an organ out of me and put it into you? Is there really a high chance that there would be a very vigorous immune response?
Hannah - Yeah, that's exactly right, Chris. In humans, we know that we should, if we take a graft and we do a kidney transplant or something like that, we should get a very big and damaging immune response to that graft. But in the Tasmanian devil, we don't see a protective immune response to the tumour. In fact, we hardly see any immune response at all and I've been interested in why this is the case. And our most recent work, we've actually shown that in the surface of the devil facial tumour disease (DFTD) cells...
Chris - Those are the tumour cells themselves.
Hannah - Yeah, the tumour cells themselves have actually changed some of the molecules that are on the surface of these cells and because they've changed these molecules, that means that they're invisible to the host devil immune system.
Chris - Alright, so are these the same molecules that in my cells, my immune system is looking at those molecules to see whether that cells, one of my cells, or if there's a virus in there, that kind of thing?
Hannah - Yes, that's exactly right. They're called MHC molecules or sometimes histocompatibility antigens and these molecules are found on the surface of nearly all cells, and they are the immune system signals. So, they send up flags to say that, "No, this cell is healthy. Don't attack this cell or to say this cells is infected by a virus or it's malignant, or it's foreign, it's non-self, attack it!" And actually gives an attack signal by the immune system. So, by downregulating these molecules by the DFTD, by the tumour cells, it's invisible to the immune system.
Chris - There are lots of viruses that use a similar sort of trick to buy themselves time so that the immune system doesn't realise there's a virus in this cell, but the immune system has kind of thought of that because it looks for cells that don't have these molecules and says, "Aha! They've been turned off, so there must be a virus in there" so they attack it anyway. So, why doesn't the immune system then say, "Aha! Your tumour cells have got none of these molecules"? They must have something wrong with them and wipe them out.
Hannah - Unfortunately, for DFTD and the immune response to DFTD, we don't understand fully that question yet. So, MHC molecules interact with one part of the immune system called T-cells and they signal to T-cells that either a cell is unhealthy or healthy. What you're talking about is done where there's a downregulation of these MHC molecules either by a tumour cell or by virally infected cell. That sends a trigger to what we call NK cells to attack the tumour cell. And we don't actually understand yet, why NK cells don't attack the DFTD cells, but that is something that we are working on.
Chris - But one thing that you presumably have got to the bottom of, if you now know these molecules aren't there is, why they're not there. So, what's responsible for causing them to disappear from the cell surface?
Hannah - Yeah, that's right. So, in cancers, we often have what we call  hard mutations in cancer genomes or soft mutations. Hard mutations occur in the gene and they're actually, usually, like a deletion in the gene or some sort of change in the gene that means that that particular gene it's not expressed. Now, when we went and looked at the genes that encode NHC molecules in the DFTD cells, we found that we couldn't actually find any hard mutations. So, we couldn't find any structural changes in these genes that would explain why they're not expressed in the DFTD cells. So, this was a bit of a mystery, but what we found is that it seems as if these are what we call soft mutations or epigenetic changes on the genome that's allowing the downregulation of these genes by the DFTD cells.
hard mutations in cancer genomes or soft mutations. Hard mutations occur in the gene and they're actually, usually, like a deletion in the gene or some sort of change in the gene that means that that particular gene it's not expressed. Now, when we went and looked at the genes that encode NHC molecules in the DFTD cells, we found that we couldn't actually find any hard mutations. So, we couldn't find any structural changes in these genes that would explain why they're not expressed in the DFTD cells. So, this was a bit of a mystery, but what we found is that it seems as if these are what we call soft mutations or epigenetic changes on the genome that's allowing the downregulation of these genes by the DFTD cells.
Chris - This is where you add chemicals or remove chemicals from the proteins that the DNAs wound around or sometimes the DNA itself to affect whether or not genes get turned on or off. But you can look for those, can't you? So, if you go hunting and look at the genes that encode these immune molecules, are they epigenetically different to a normal cell?
Hannah - That's what we're looking at at the moment and it does seem as if they do have some epigenetic changes in these genes that's actually silencing the genes, causing them to be turned off. And if we treat the DFTD cells with epigenetic modifiers, we can actually increase the expression of these genes again. Actually, that's probably one of the most exciting things about this finding, is that because these changes are what we call soft changes or they're reversible, we can actually in the lab, restore the expression of these MHC molecules to the DFTD cells. We think that that could have really important consequences for developing a vaccine to the disease.
Chris - Because you'd give them a drug that turns these molecules back on, enabling the immune system to see the cancer which was previously cloaked in this sort of invisibility shield. It would come back to the attention of the immune system which will then presumably deal with it?
Hannah - Exactly and an alternative is that we can, by treating these DFTD cells in the lab, we can engineer them to express MHC molecules on their cell surface, and then we can put them back into the devils. So, we're putting back in DFTD cells that have a signal to the immune system of, "We're unhealthy. We're foreign. You need to attack us."
Chris - This must be music to your ears, Elizabeth?
Elizabeth - Yeah, very, very exciting work.
Chris - How do you see this being applied?
Elizabeth - Well, we're all hoping that perhaps this will lead to a vaccine that can be delivered in the wild to protect devils from succumbing to this disease.
Chris - Do you think it's realistic?
Elizabeth - I think so. I think there's still quite a lot of research still to be done, particularly on why it is that the tumour can get established in a devil that's got a healthy immune system. And I think that understanding in more detail how the immune system is not able to attack it will be very important before we can develop a vaccine.
Chris - And Hannah, what can we learn about ourselves and possibly doing better organ transplants from studying what is going on in these devils because they're effectively doing an organ transplant that is not being rejected, aren't they?
Hannah - Yeah, that's right and there's that aspect of it and there's also the fact that this is a cancer that is a master at invisibility. And so, not only is invisible in a single animal, but it's invisible each time it's being passed through into a different individual. And so, the mechanisms that it's evolved in order to escape the immune system are very, very sophisticated. And so, I think we can learn a lot about how a cell is immune privileged and how it can disguise itself from the immune system.
Hannah Siddle and Elizabeth Murchison, both from Cambridge. Thank you very much.
47:08 - Cancer and Immunity
Cancer and Immunity
with Richard Wells, Cambridge University
The majority of cancers are not transmitted but arise spontaneously in the body. So, why doesn't the immune system deal with that? This is the question that PhD student Richard Wells is looking into at Cancer Research UK's Cambridge Research Institute.
Richard - So, our lab is trying to work out why it is that the immune system can't stop cancers from growing in people. It's been known for a very long time that tumour cells, these rouge cells that grow very fast and in a totally inappropriate way. They're so unusual and they're so strange that immune cells that normally are looking for bacteria and viruses, and that sort of thing actually realise that these cells are broken in some way and that they shouldn't be there. But, obviously, the unfortunate thing is, that people with a very healthy immune system still get cancers. So, there must be a way that tumours are trying to escape that?
Chris - Why should the immune system attack the tumour because they are after all your own cells?
Richard - That's a really puzzling thing about it. When this was first suggested in the '50s, people thought those who'd raise the idea that they were mad because you know, these are human cells. But it looks like the cells are so stressed and so damaged that they might start producing molecules that act as a trigger. In the same way that if you were to cut  your arm, it goes red and hot, and that's immune cells going in. And the reason that they go in there is because that they're dead and dying cells, and that also is something we see in cancers. There are cells that are very, very stressed and they're working really hard to fix themselves, but when they do that, they're putting out all sorts of signals that say that they're not right and may not be working properly, and that also goes in immune cells. Some people have called a tumour 'a wound that never heals'.
your arm, it goes red and hot, and that's immune cells going in. And the reason that they go in there is because that they're dead and dying cells, and that also is something we see in cancers. There are cells that are very, very stressed and they're working really hard to fix themselves, but when they do that, they're putting out all sorts of signals that say that they're not right and may not be working properly, and that also goes in immune cells. Some people have called a tumour 'a wound that never heals'.
Chris - So, you should have the equivalent of the red arm that you're wounded in your cancer, but you don't. So, something unusual is going on. The cancer is in some way preventing that from happening?
Richard - Yes, so what we get is, we get the sort of the red flare at the very beginning that says there's something wrong, but what we never get is that they will fix it and we get rid of what's wrong. And that's the bit where - there are sort of two bits to your immune system and the first bit is the cells that come in and makes it red, and makes it inflamed, and says, "Look, there's something broken here. There's something not right." Those guys coming into cancers and they're doing their job really well. Unfortunately, the second set of cells that's supposed to come in and kill off anything that's broken, they aren't actually doing their job.
Chris - It's not that a cancer is a mixed bag. There are lots of cells, some more damaged than others, and some of them do get deleted in this way, a bit like you're saying, the immune system comes and then whacks those out. But then there are other cells, a little bit more normal at least to start with, maybe the cancer gets away with having those cells, the immune system is prepared to let them go. Is that possible?
Richard - So there's two big theories as to how these tumours might be getting away with what they're getting away with. Some people think the tumour cells manage to make themselves look quite normal and the ones that look the most normal don't get killed. But actually, although we all think of the tumour cell, the rouge cell, it's dividing; actually, they pull in loads of other different types of supporting cells.
Chris - What? They attract those cells to come in to the cancer from elsewhere?
Richard - Yeah, so it's exactly the same again as like a wound. In a wound, it pull in all of these networks of cells, they form a mesh around and help the wound to fix itself. And what we found is that one of them, it's very, very potent in its manner of stopping immune cells from working.
Chris - Wow! So, the cancer is recruiting a totally different non-cancer, a healthy cell to come into the cancer, and that cell is in turn, turning off the immune response?
Richard - That's absolutely right.
Chris - So, you get these local immune control or suppression really where the cancer is, allowing it to escape under the immune radar, and the immune system ignores it?
Richard - Absolutely, yes. And there's only one type of all of these types of cell and it seems to be, one of them is enough to stop the second type of immune response that's very, very specific, and the cell it longs to kill.
Chris - Why doesn't my sore arm recruit those same cells and turn off the immune response? Why is it only in cancer that this happens?
Richard - So, it seems to be that if you're trying to fix a wound, there's a certain amount of sort angry immune cells coming in which is a good thing because it cleans it up. It gets rid of everything, but there has to be a flipping point where you start to say, "Okay, I've had enough sort of angry cells coming in and I have to try and calm this all down. I have to try and make everything go back to normal and it can fix itself." And what we get in the tumour is that you get the angry bit, but it then lives for many, many years in a state where everything is calming down and sits there, growing within a network that's supposed to just be there for a few days, weeks, something like that.
Chris - Can you get rid of these cells?
Richard - So, we can in animal models at the moment and that's proven to be very, very effective and we can actually completely control tumour growth of pancreatic tumours in mice. And these tumours are very, very difficult to control with drugs and in human patients, the survival of pancreatic cancer is very, very poor. It's harder to translate that into a human patient because what we've since worked out is that, as I said, these are totally normal cells doing a normal thing. And they seem to have really important roles in all tissue. So, the approach that would be the obvious one was to say, "Let's just get rid of these cells." Actually, unfortunately, it's very hard to do and will cause real problems for a patient. But what we're trying to work out is precisely what in the tumour makes them different. Can we find a drug that's really specific to stop what they're doing in the tumour?
Chris - Or stop them going into the tumour in the first place?
Richard - Indeed, so that's the second sort of way that we're also looking at saying, where do they come from and how are they getting there. It's possible that there's a mixture. Some get pulled in from the bone marrow, so the bone marrow tends to be this big factory that produces all sorts of different types of cell that go around the body. And we think some of them go from as far as the bone marrow, all the way through your blood and into your tissue.
Chris - What are these cells called?
Richard - So in general, the cells that support a tumour are called stromal cells. They're sort of a network. So, what we call this cell is the FAP positive stromal cell because we found that the protein that marks it very specifically is FAP (fibroblast activation protein).
Chris - And if you look at a lung cancer, is it equally likely to have these cells in it as a bowel cancer, a pancreatic cancer, or even a brain tumour?
Richard - So, there's different subtypes of tumour and the one that we're looking at is adenocarcinoma. It's this one type of carcinoma that comes from glandular tissues and if you look in human adenocarcinomas, the FAP positive cell is present in almost all of them.
Chris - An amazing thing to be working on during your PhD which could culminate actually a really effective treatment for cancer. Is that not awe you a bit? Or sort of blow your mind to be thinking you're working on something that is really that cutting edge therapeutically?
Richard - Yeah, it's very exciting. I mean, particularly as I'm training to be a doctor as well. It's really great to see work that's coming through that can operate both at the basic science of it, but to see where it's going and to see that it could help patients is fantastic.
PhD student Richard Wells.

54:14 - Why don't humans have tortoiseshell hair?
Why don't humans have tortoiseshell hair?
Ian - I'm Professor Ian Jackson at the MRC Human Genetics Unit in the University of Edinburgh. We understand a bit about the genetics of human colour. We know the gene that causes red hair for example and we know some of genes make long versus dark hair. But in humans, we never see coloured patterns in their hair.
Now in cats, and many other mammals, we see a whole range of patterns. Cats have this stripy tabby pattern. So the stripes are caused by a gene called amino peptidase q and when this gene is missing, the cats lose that stripy pattern and have a more blotched pattern where the stripes become irregular. We humans have this amino peptidase q gene, it really isn't going to do the same thing in humans. We don't actually know what it's doing.
Cats also have that tortoiseshell or calico pattern. That's caused by a gene on the X chromosome that makes orange pigment and females have 2 X chromosomes. Females shut down one of the X chromosomes in random in different cells. So, if she has 1 orange X chromosome and 1 black X chromosome, then you get a mixture of orange and black patches. It's the classic tortoiseshell pattern and tortoiseshell cats are always female.
Hannah - Also, the pointed pattern in Siamese cats where the ears, nose and extremities are darker stems from a mutated colour gene that switches off at higher body temperatures.
So, humans seem to have simple genetic rules for hair colour and they don't respond to the patterning genes in the same way as cats, which is why you don't generally get a tabby human.
There are exceptions to this though. People with piebaldism do show patches of white hair even at a young age. Their melanocytes, so the cells that produce the pigment for hair colour have been mutated and switched off, and it's this that produces the colourless patches.









Comments
Add a comment