From the beauty of a sunset or the ugliness of war to the smile on a loved one's face, our eyes bring us all kinds of information about the world around us. now researchers are working to develop new therapies for people who have lost this precious sense. Plus, smelling elephants, marmoset twins, and an all-seeing gene of the month.
In this episode
01:03 - Seeing the Light
Seeing the Light
with Prof Rob Lucas, Manchester University
Poets may claim our eyes are the windows to the soul, but to scientists they're an important way of sensing the world around us by detecting light - or seeing, as we usually call it. To find out more about how organisms, including humans, detect light - through special cells called photoreceptors - Kat Arney spoke to Rob Lucas, professor of neurobiology at Manchester University.
Rob - There are lots of different mechanisms that organisms use to sense light. Probably because it's going to be amongst the oldest sensory systems that organisms evolve. But if we concentrate on animals and particularly on vertebrates then there is a particular class of proteins called opsins that they use to detect light. And these are proteins that bind a derivative of vitamin A called retinaldehyde. It's actually that retinaldehyde that absorbs the light and the opsin protein has the really clever job of detecting that light absorption and setting in chain a biological response. The rods and cones are packed full of opsins that are used to absorb light.
Until about 10, 15 years ago, it was assumed that they were the only cells in the retina that contained opsin and they were the only cells therefore that were capable of responding to light. And so, that's still the major way in which mammals detect light. But we've been interested in this new sort of cell type in the retina that also contains opsins and we've discovered them about a decade ago or something like that.
Kat - How did you find these?
Rob - So, that was work not just from my own lab, but from various labs around the world. My own contribution to it when I was working as a post doc in Russell Foster's lab, we generated mice that lacked rod and cone photoreceptors and therefore, by all rights should have been completely blind. But it showed that they still had some light responses and that implied that there was something else in the retina capable of responding to light.
And then a scientist at Brown University in the US called David Berson recorded electrophysiologically from some of the retinal ganglion cells, these are the cells that form the optic nerve, and showed that they were capable of responding directly to light. That was first description of this new photoreceptor.
Kat - That must've been incredible to think that there was something as fundamental as the eye and there's this whole extra layer that we never knew about.
Rob - Yeah, it's been really, really exciting and certainly, at the time when I started working in this field, there was huge reluctance from vision scientists to countenance the idea that they might have missed something. In a way, it's quite a nice illustration of general themes I think in biology going forward and that is, that the more you look at things in detail, the more you find rare events that are nonetheless really, really important.
So, this sort of photoreceptor in human retina, there might be about a thousand of these photoreceptor cells compared to many millions of rods and cones. So, if you were to just - as a first approximation, say, where does photoreception occur in the mammalian retina? The answer is in rods and cones. But that's not to say it doesn't also occur elsewhere. Just because there were so few of these other cells doesn't mean they aren't important for us. So, there's a lot to be discovered by looking at rare events.
Kat - And of course, this begs the question, what are they doing there?
Rob - Why are they important? Our knowledge of that is really developing but their really widely accepted roles to provide our brain with a signal of the overall amounts of light there is in the environment. So, why do we need that? The most obvious answer to our question is that we use it to adjust our physiology and behaviour according to time of day. And so, we used the output of these cells to synchronise our biological clocks, time of day. So, when you fly to New York, its light has detected by these new photoreceptors which tells you that it's daytime when you're expecting it to be night and therefore adjust your clock. But also, there are direct effects of light. There are alerting effects of light and this come probably mainly from these retinal ganglion cells.
Kat - Waking you up.
Rob - Yeah, waking you up, increasing your body temperature, changing hormone levels - from that to very simple reflex responses. So, for example, the pupil light reflex. Many people will be aware that when the light is bright, your pupil is small. All that's down to these ganglion cells as well.
Kat - And what do we know about the way that these unusual photoreceptors are working? Do they have similar molecules in them to the rods and cones?
Rob - They also use opsins, but it's a particular sort of opsin. It's an opsin called melanopsin. It's called melanopsin because it was first discovered in the dermal melanophores. So, these are the skin pigment cells of amphibia. And so, it's involved in changing skin pigmentation in amphibia, depending on light intensity. And it's this opsin which has been involved in those skin cells of toads and frogs that is then also present in our eyes and we use to detect light.
Kat - How widely across the animal kingdom do you find these unusual opsins and are they always used for detecting general levels of light and then doing something in response?
Rob - So, it turns out that actually, mammals are unusually boring in terms of the range of opsins that we have. So, we have in addition to our rod and cone opsins, we have melanopsin. We use that as I've described. If you look outside mammals and other vertebrates, they have melanopsin, they have rod and cone opsin, they also have lots of other sorts of opsins that they use for exactly this purpose. And very commonly, those opsins would be found in places other than the eyes. So, we talked about the amphibian skin, but also, deep within the brain of many of these other animals, they have photoreceptors which means that if you were to remove their eyes for example, they would still synchronise biological clocks to local times, still adjust their behaviour according to light dark cycles.
Kat - That's like some freaky third eye!
Rob - Yes, so the third eye, parietal eye in lizards is obviously photoreceptor but then they also have pineal glands which we have a pineal but it's not photosensitive. But in these other organisms, it is photosensitive. The more that you dig around in these guys, the more you find light sensitivity to the extent that if you take for example a fish heart and put it in a culture dish, you can get it to respond to light.
Kat - Why on earth are they so widespread then through evolution?
Rob - One of the first pieces of advice I had when starting studying biology is never ask a 'why' question because we can never know the answer. But I'm going to answer it anyway. Of course, it's complete speculation. The question of why they're so widespread, I guess the answer to that must be that this light information is valuable for lots in different parts of our bodies, right? So, the fact that you have photoreceptors in the heart is because the sort of level of activity you have in the day and night is different. And so, it's useful for the heart to know what time of day it is. If we can do that by having its own light measurement system, why wouldn't it?
Kat - At least if you're a fish.
Rob - At least if you're a fish. But even if you were a mammal, why is that not the case also for a mammal? So obviously, there are lots of small mammals that light would penetrate their body just fine. And so, you can break it down into those related questions, why is it valuable for a fish let's say, and why isn't it valuable for a mammal? And I think we can say what might be valuable for a fish. Why it's not valuable for a mammal, it's harder to answer. The current best guess on that is that actually, it's a reflection of our evolutionary history.
So, mammals, as far as one can tell would nocturnal for tens of millions of years of our evolution. When they're nocturnal, there's not much light around to be exposed to. As a result, lots of these light-sensitive proteins are lost because the light doesn't reach the heart of a nocturnal animal as much as it would for a diurnal animal for example. And so, lots of those photoreceptors were lost and that's why we don't have them now even though many mammalian species could if they wanted to have photoreceptors outside the eyes.
10:09 - Maths and Reading
Maths and Reading
with Prof Robert Plomin, Kings College London
In the news this month was a new study in the journal Nature Communications from Professor Robert Plomin from Kings College London and his colleagues, showing that gene variations linked to reading ability in children are also linked to maths skills. Kat Arney asked him to explain a bit more about the motivation behind the study, and what it might mean.
Robert - What I've been interested in is the extent to which the same genes affect different traits. It might be surprising to listeners to know that diverse cognitive abilities, mental abilities like spatial ability and verbal ability, memory, as well as learning abilities like reading and math are all highly heritable. That means there's a lot of genetic influence. The reason why one person differs from another, the majority of that answer is genetics.
Kat - So, you get it from your mum, your dad.
Robert - Yup, you inherit it in inherited DNA differences in DNA sequence. So, people might be surprised to know, but it's no longer interesting to ask that question - is it heritable - because every single study over decades has shown that it is heritable. So, we're trying to go beyond heritability.
One of the most interesting questions is to ask, are the same genes affecting different traits? Because you might expect something like reading is very different from math because the cognitive processes in doing math and reading would seem to be so different. So, you would expect that although they're both heritable, different genes would affect the two skills.
Kat - So, you've got like a maths gene that means you can add, subtract, juggle numbers in your head, and a reading gene that means you can ocus and understand words.
Robert - Yeah. Most definitely, what's come out of the molecular genetic work on DNA is, it isn't one gene. We're talking about hundreds or maybe thousands of genes of very small effect. So, the question is more quantitative. To what extent are the different genes that affect reading, overlapping with the genes that affect math? The punchline is that although you might expect very different genes to be involved, in fact, most of the genes are the same.
Kat - How did you actually find this out, that the abilities in reading and maths are heritable in this way and that they do seem linked to similar genes?
Robert - The cool thing about this study, it was one of the first to use this totally different method that doesn't use twins or siblings or family members that uses unrelated individuals and genome wide DNA similarities. So, there are these chips that genotype your DNA on something the size of a postage stamp and it gets a million of these DNA markers. So, we can use those million DNA markers to say, "Are you and I a little more similar than me and someone else" than to say, "Are we a little more similar in our reading ability than other people?"
So, instead of identical versus fraternal twins, pair by pair for thousands of pairs, we ask, "Are the people who are genetically more similar at a DNA level - are they more similar on a trait-like reading or on the covariance, the relationship between reading and math?" With 3,000 unrelated individuals in our sample, that gives you nearly 5 million pair by pair comparisons if you see what I mean. So, it's very powerful as well.
Kat - So, you're finding that out of all these people, there are relationships between reading and maths ability and certain parts of the genome, is it possible to say, "Right, it's that gene, it's that gene, it's that gene"? What can this tell us?
Robert - Yeah. Well, that's the big prize. People want to discover these specific genes that account for these heritability. The problem is, throughout the life sciences, biomedical sciences, complex traits like these and common disorders, not rare single-gene disorders, they're influenced by many, many genes of small effect. And so, throughout the life sciences, it's been a major disappointment that although many things are heritable, it's very, very difficult to find the specific genes involved because they each have such small effects.
So we're going to continue trying to find the genes. There's a lot that's still happening. For example right now, the big development is sequencing all 3 billion base pairs of DNA. So, that's what people are doing now. That's the end of the story in terms of DNA sequence variation because you've got all 3 billion base pairs. Not a million DNA markers but 3 billion sequences of DNA. So, that's one direction that things are going.
But I think just at the level we're at now, if there are any parents who still think children are a blob of clay that you just mould to be what you want them to be, tabula rasa, 'the blank slate'. They might pay attention to this data and realise that kids aren't just moulded to be what you want them to be. Maybe as parents and teachers, we need to recognise that children differ even very early in life and maybe respect those differences to a greater extent.

15:00 - Smelling Elephants
Smelling Elephants
When you think about nature's champion sniffers, you probably think of dogs, whose noses are incredibly sensitive. But a new study published in the journal Genome Research shows that they may have competition. Researchers compared the number of olfactory receptor genes in 13 different mammalian species - these are the molecules in the nose that detect different odours - and discovered that African elephants have the largest number, clocking in at around 2,000. This is twice as many as dogs and five times more than humans.
As part of the study, the scientists also discovered more than 10,000 olfactory receptor genes in total across all the species, but that each animal's repertoire was pretty much unique - only three receptor genes were shared across all 13 species. The diversity has arisen thanks to hundreds of genes being copied and lost during evolution, and highlights the differences in the sense of smell across the different animals.

15:55 - Marmoset Genome Revealed
Marmoset Genome Revealed
An international research team has unveiled the complete genome sequence of the common marmoset - a small monkey found in South America - publishing their work in the journal Nature Genetics. It's the first genomes from a so-called New World monkey, which is further away in evolutionary terms than primates such as gorillas, chimps and humans, helping to shed light on primate evolution and biology.
One of the most interesting aspects of marmosets is that they tend to consistently give birth to twins, unlike humans and other primates. The scientists discovered that a gene called WFIKKN1 seems to be playing an important role in twinning, and might help to explain multiple pregnancies in humans. The team also discovered intriguing differences in growth hormone genes, which may explain their small body size, as well as a number of interesting microRNAs - small molecules that help to control gene activity.
The researchers hope that the new genome sequence will open up new research avenues, particularly related to reproduction, which could shed light on human health and disease.
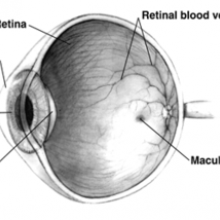
17:09 - Restoring Sight
Restoring Sight
with Rachael Pearson, UCL
At University College London's Institute of Ophthalmology, Dr Rachael Pearson and her team are developing ways to restore sight to the blind, by replacing damaged photoreceptors in the eye. Kat Arney started by asking her why we can't just grow new photoreceptors ourselves.
Rachael - The problem with most neuronal cells, certainly in mammals including humans, is that we're very bad at regenerating our neurons and that goes to the photoreceptors as well. So, once they've degenerated, once they've died, we're not able to replace them. There are certainly other animals that can regenerate. So for example, fish and a lot of the lower vertebrates. So frogs, fish, newts, all of those sorts of animals have an amazing capacity to regenerate. In the case of the eye, you can ablate large areas of the eye and they will actually grow a new retina.
Kat - You can poke them in the eye and it will grow back.
Rachael - You can poke them in the eye and they'll get a new one. Sadly, it's not the same for us. We're much worse at doing that.
Kat - If we can't grow back our photoreceptors, how are you and your team trying to counteract some of this degeneration that happens?
Rachael - So, there are various strategies that are being looked at obviously. One is that you try and get in there first and you correct the genetic defects - that would be gene therapy. And then there are cell therapy strategies which is the idea that we've lost those cells. Can we put them back? Can we find a suitable cell type that will do the same job? And then related to that is, could we actually try and persuade our own retina to regrow and repair itself?
But coming back to the cell based therapies, which is the area that I'm primarily working on at the moment, we're interested in trying to identify appropriate sources of donor cells that we can literally, physically transplant back into the patient's eye. And then the hope is that those cells would then migrate to the right place, they turn into the right cell that we're interested in - the photoreceptor. But then they also need to do quite a few other things. They need to wire up. They need to form new connections to those next cells in line and they need to be able to detect light and pass those signals on.
Kat - Where are you with this kind of research? What have you done so far?
Rachael - All of the work to date is still very much in the preclinical stages. We're not at the clinical trial stage yet. But what we have managed to do is demonstrate that it's possible to take cells from one eye and you essentially dissociate them. If you turn them into single cells, you're not putting a piece of tissue in. but you can immature cells from a developing retina and you can dissociate them into single cells and then transplant those into the back of the eye of a recipient animal. That recipient has a retinal degeneration so its photoreceptors are dying.
If you transplant those cells and these cells manage to migrate into the degenerating retina, they can turn into the right cell type into the photoreceptors and they can also form new connections. We've then been able to go on to show that those cells are able to function as a normal photoreceptor so they are light sensitive. They detect light and they turn this into an electrical signal. This signal is then passed down both through the retina, but then also, all the way up to the brain.
Kat - Sounds good.
Rachael - It sounds good. It is good. It's very encouraging. So, that's where we've got on that side. One of the issues with it is that those cells that we identified as being really good at doing this come from a period in development that would make it very difficult for us to translate into the human situation, because it comes during the equivalent in humans would be towards the end of the first trimester, second trimester.
Kat - This is foetal tissue?
Rachael - So, this would've then be in foetal tissue which obviously has ethical and practical implications. So, we want to then think about alternative donor cells. And so, that's then led us onto stem cells themselves. We're now trying to take embryonic stem cells and basically grow eyes in a dish.
Kat - These are the stem cells from the very, very earliest time of development where you have a little ball of cells when they first start get going. How do you then turn them in a dish into these photoreceptor cells?
Rachael - That would be giving the secrets away. Essentially, what you're trying to do is recapitulate normal eye developments. We're very lucky working in the eye because it's a wonderful model and it's been studied for many, many years. So, we actually know a lot about how the eye normally develops. We know a lot about the signalling pathways that turn - a cell that could grow and be an eye or equally, it could grow a liver and kidney or anything else. We know what the signals are during embryonic development that say actually, "No, good. Don't do that. Go and be an eye or go and be a cell within the eye."
And so, we're able to start to use these and introduce the same signalling pathways in the culture dish. So, we're trying to stepwise turn this cell from being an undifferentiated embryonic stem cell into a cell that knows it's going to become a retinal cell and then more specifically, it knows it's going to become of these photoreceptors that we have found to be really good for transplanting.
Kat - And that's using embryonic stem cells which still you'll require source of embryos for them. Is there any interest in using the new kind of reprogrammed stem cells, the inducible pluripotent stem cells we hear so much about?
Rachael - Absolutely, there's interest in those. We're investigating them as well. We're very interested to understand their potential. They have the advantage that obviously, you could take them from an autologous source, which means it's coming the patient themselves. So, you remove the ethical issues associated with embryonic stem cells. The problem with those obviously is that an IPS cell, if it comes from a patient with a genetic mutation that causes retinal degeneration, any cell that we turn into retina from that patient will still have that retinal degeneration.
So, we can make new photoreceptors but they will still be affected in the same way. We have to think about that when we're using IPS cells and we may need to go and correct that genetic defect in vitro before we use those cells for cell transplantation. So, it's not completely straightforward to use an IPS cell. But they're still very interesting sources. I think at the moment, the field is at a point where we still need to investigate both and look to see the relative pros and cons of both of them.
Kat - With the kind of the molecules, the pathways you're discovering that turn embryonic stem cells into photoreceptors, are there similar pathways that could be reactivated within the eye to make new photoreceptors grow in situ in the eyeball?
Rachael - Yeah. That's a really interesting strategy and it's one that I'm interested in. I think it's still very much in its infancy at the moment as an idea. So, there is the idea at the moment that there's a population of cells called monoglial cells and these are support cells in the eye. They in lower vertebrates can de-differentiate, which means they can kind of take step back with developmentally. They can become these cells that proliferate to generate new neurons and they can help also at the repair process. So, the idea might be that in humans, maybe we can do the same thing. We don't know yet, but the idea is that we might be able to turn these smaller cells back a step and allow them to re-enter the cell cycle and generate new photoreceptors. But that's very much at its infancy at the moment.
Kat - It sounds like there a lot of interest, a lot of excitement, a lot of ideas, but presumably also some very big challenges. What do you see is maybe the key challenge that needs to be overcome if you can pick one to really take this forward?
Rachael - As you said, there are many challenges. And so, it's tough to know which one is going to be the defining one. I think at the moment, so much of our work has been based on animal models and particularly on the rod transplantation. So, as I mentioned those are the ones that we're interested in that we use to detect low light levels. For us as humans, obviously, the really important things are cones. We rely so heavily on our vision to navigate during the day. I think the most important challenge really is to know whether or not this strategy would work for cones. So, that's something that we're putting an awful lot of time and effort into at the moment and trying to see if we can transplant cones and restore cone mediated vision.
Kat - It must be really fantastic when you could see that you can transplant these cells and that they will work, and that they will send signals. It's difficult to cast too far into the future, but it's really nice to think of maybe in sort of 15, 20 years' time that there are viable cell therapies. That would be great.
Rachael - It will be great. It will be absolutely amazing and that's obviously what keeps all of us going. We really do hope that this strategy will come to fruition.
Could we make all mosquitoes one sex?
This is a great idea, and exactly what Dr Nikolai Windbichler and his team at Imperial College, London, are trying to do. Kat Arney asked him how... Nikolai:: Well, the idea is if you progressively shift the sex ratio of a population towards males and there are fewer and fewer females in the population then the overall size of the population will decrease up to a point where the population cannot sustain itself any more and will actually crash.
Kat:: What did you do to try and skew this sex ratio?
Nikolai:: So, we introduced into the mosquito a gene which essentially destroys the X chromosome. As you know, as in humans, also in mosquitoes, there are two types of sperm produced by males: sperm that carry the Y chromosome and sperm that X chromosome. The sperm that carry a Y chromosome produce sons. The sperm that carry X chromosome produce daughters. So, we found a way to specifically eliminate the sperm that carried the X chromosome so that only the sperm that carried a Y chromosome would be functional and would make these males produce only sons. This is a very promising technology but we're still many steps away to rolling this out. We have both technical hurdles to overcome still, but also have to make sure that all aspects of biosafety, safety, ethical concerns and regulatory concerns would be addressed before we go any further with this. The next step is to take these mosquitoes and test them at a larger scale. We have tested the technology in small population cages, but we have a facility in Italy where we have larger cages that are a more field-like control environment. And there, we want to test the technology to see how it performs.
Kat:: Thanks to listener Lorne Henry and Dr Nikolai Windbichler - and there's a longer version of that interview on the Naked Scientists website. If you've got any questions about genes, DNA and genetics, just email them to me at genetics@thenakedscientists.com.
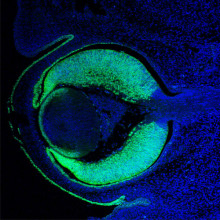
28:38 - Gene of the Month - Pax6
Gene of the Month - Pax6
with Kat Arney
Kat - And finally it's time for our gene of the month, and in keeping with our theme of vision it's Pax6. Known as PAX6 in humans and eyeless in fruit flies - but found in a wide range of different animals including mammals, insects and fish - the gene is a 'master controller' telling a developing embryo exactly where to grow an eye. Impressively, the Pax6 gene from mice can be put into fruit flies, and can direct them to grow fruit fly eyes. And as might be expected, any faults in such an important gene lead to major problems with eye development, so researchers are studying the gene in detail to gain insights into why some children are born blind.









Comments
Add a comment