In this episode of the eLife podcast we hear about the spread of the Ebola virus, the financial costs of research misconduct, aging in yeast, grooming in flies, and symbiosis between bacteria and fungal cells.
In this episode
00:36 - The spread of Ebola
The spread of Ebola
with David Pigott, University of Oxford
Changes in lifestyle, such as increased human connectivity and movement, mean that new outbreaks of Ebola virus are likely to be very different to previous outbreaks. Chris Smith spoke to Dr David Pigott, from the University of Oxford, about why the 2014 outbreak was so much larger and deadlier than before...
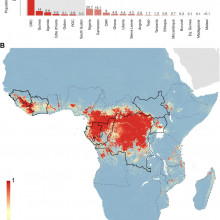
David - Given the recent outbreak in Guinea, we've been looking at how Ebola is actually spread around the African area. So, when you consider Ebola, it's important to recognise that there are two distinct processes going on. Firstly, there's what we call the zoonotic phase where there's cycling of Ebola viruses between different animals and we currently think that bat species are the main reservoir of this, but also great apes such as gorillas and chimpanzees can also be infected. Now occasionally, humans interact with this animal population, normally through bush meat hunting or butchering of animal carcasses and then it transitions from animals to humans, and then there is subsequent human to human transmission going on. So, in the outbreak in Guinea at the minute, genetic evidence has shown that there's only actually been one transmission event from animals to humans and that all subsequent cases are actually human to human interactions, so, burial practices, or caring after sick relatives for instance, or within the hospital setting itself.
Chris - What can you tell us about Ebola's history, because this is not a new infection, it's been knocking around and periodically surfacing for a number of years, isn't it?
David - Yeah, so, it's got about a 45-year history. In 1976, there was a series of unexplained symptoms that had never really been recognised before that occurred simultaneously in what was then called Zaire, so, Northern Democratic Republic of Congo and also in the southern borders of Sudan. And since then, we've seen small numbers of cases, no more than 400 to 500 individuals involved in an outbreak and spreading no further than one or two imported cases elsewhere in the world. So, with the Guinea outbreak, what we've actually seen is something that seems completely different to the previous history of Ebola. We've had more individuals infected than all other outbreaks of Ebola combined, and we've actually seen it spread to more countries than we ever have before. So, it's moved from Guinea to Liberia, Sierra Leone, and then through air travel to Nigeria and Senegal recently.
Chris - So, would you say then that the sort of dynamics, the way in which the disease is behaving, in this outbreak are clearly different than patterns you've seen in previous years?
David - So, in the work that we've been doing, we've been looking at how the connectivity of these populations has changed dramatically over the last 45 years. The population size themselves has nearly tripled and actually we only have international air connectivity data for the last five years or so, but within that period of time, some countries have actually increased their outbound flights by three or four times, so it's a pretty rapid increase.
Chris - And why do you think that those things changing, could increase the likelihood that Ebola will surface and alter the way in which the disease behaves in communities, like it has?
David - All these human changes are actually going to be very important in how we see human to human transmission of the disease. So, increasing population size, increasing population density, is going to mean that if individuals are infected, they're more likely to come across others to infect them.
Chris - So, how did you go about doing this study? How did you get the data that's enabled you to begin to look at how the disease might change or how there are various factors that could influence the dynamics of this infection?
David - So, we looked at the last 45 years or so of published articles on Ebola virus, both within humans and animals, and basically, it was a bit of detective work identifying human outbreaks and then backtracking it all the way to see who was the first person that got infected and, if possible how they became infected.
Chris - And what implications are there? On the basis of what you have found in your analysis, for both preventing future episodes and outbreaks of Ebola but also perhaps managing better the current crisis we've got.
David - Yeah. So, there are two different aspects to that. I think one of the most important things that our paper shows is that the range of different countries that are at risk of Ebola is actually a lot greater than we first thought. So, we predict that there's 22 countries in Central and Western Africa that there's the environmental possibility that animal to human transmission can occur. Seven of those countries, we've actually seen that transmission occur but there are 15 countries that haven't actually seen Ebola in those areas. So, these would be great places to start surveying bat populations or other mammals that could be reservoirs of Ebola, i.e. animals that are infected with Ebola but don't show signs of infection, so they don't die necessarily, to get a better understanding of how the disease is cycling within animal populations. And that's a very important process to understand because then it allows us to assess the risk of, does hunting in this area actually mean that the transmission from animal to human could occur?
Chris - Now given that you've identified the potential for Ebola outbreaks is much bigger than the present pattern of activity, what sorts of strategies would you urge apart from obviously monitoring to try to prevent this happening in future or to get a handle on where it might happen and mobilise some sort of resource to stop it?
David - So, I think it'd be a very tall order to try and say, "Oh, the next area where Ebola is going to transition from animals to humans is going to be...," but one thing that we could do is look to see how these populations are connected. So, there are some people that use, for instance, mobile phone data to see how people move within a country. So, if we can identify areas that are very well connected, we could perhaps prioritise those transport nodes, as it were, to prevent further spread of any infection, should it actually occur.
07:08 - Paying the price
Paying the price
with Ferric Fang, University of Washington
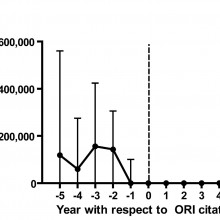
A set of 149 papers that were retracted due to misconduct had been funded to the tune of $58m by the NIH.
Dr Chris Smith spoke to Ferric Fang from the University of Washington about not only the financial impact, but also the damage it can cause to the reputation of science overall...
Chris - All scientists follow a code of ethics and good practice, which says that the results that they publish must be true and accurate. Regrettably, we know that this isn't always the case. So, how much does scientific fraud actually cost? Ferric Fang has been looking at the impact in the US.
Ferric - With discussions of research misconduct, there's often an interest in how much public money has been wasted in research that turned out to be fraudulent. There's a case going on the in States right now involving a researcher who worked on an HIV vaccine project, and the laboratory involved had received almost 20 million dollars US in grant funds so I think the public is rightly concerned about whether their investment in science is being misspent and to what extent that's taking place.
Chris - How did you do that? How can you put a finger on work that's fraudulent? What are you using as your index of falsification?
Ferric - We looked at papers that had been retracted and we looked at the underlying causes for that. We then looked at what the grants were, that supported that work and we looked at how many other papers were supported by those grants, and then we apportioned the fraction of the grants that were attributable to that specific paper that turned out to be fraudulent and was retracted.
Chris - Just put some numbers on this for us. How many retractions were there? How big was your data set?
Ferric - The number of retractions ever in the research literature is actually pretty modest, a little over 2000 papers that had been retracted out of well over 20 million. Then we had to narrow those down further, so we were talking about articles that originated from the United States, and so, the subset of data that we were looking at ended up being 291 articles published between 1992 and then 20 years later, 2012.
Chris - As a percentage, that means that scientists are relatively well behaved, or at least put another way, when they're caught, the numbers seem to suggest they're relatively well-behaved.
Ferric - Well, I wouldn't dispute that statement. I do think that the vast majority of scientists are scrupulous and honest, but I also would say that the number of papers that are retracted for misconduct, which is only about 1 out 20,000, is really just the tip of the iceberg because there are many, many cases of misconduct which do not result in a retraction.
Chris - If you were to ask scientists to be candid and face no consequence but say to them, "Have you ever falsified data?" What proportion of scientists say then that they have?
Ferric - So, that exactly, has been done, and taken collectively, they suggest that on the order of 1 to 3 percent of scientists admit to having committed serious fabrication or falsification of data at least one time in their careers.
Chris - So that number's probably more realistic as a measure of the degree of misconduct. So, taking your present study, you're able to now put some kind of price tag, some kind of consequences on this. So, what did you find was the sort of financial impact? How much have we spent on research that turns out to be dud?
Ferric - The actual amount is really a very small amount. We came up with about 58 million dollars US out of billions of dollars that have been spent on research over that period of time. If you were to say we have to fudge that upward because 1 to 2 percent of scientists have actually committed misconduct, which I think would be a gross overestimate, we still end up with on the order of 1 and 1/2 percent.
Chris - Are you factoring in the snowball effects, because if there's some dodgy data in there and someone else then uses that to do legitimate work, then their legitimate work could be made illegitimate and therefore, a waste of money owing to the fact that it was founded on fallacious principles.
Ferric - So, you're absolutely right, but we did not try to factor that in. I think it would be very difficult, but this is one of the great concerns about fraudulent research. A good example is the research that was supposed to link vaccines with autism and intestinal disease. That paper has now been discredited, but the anti-vaccine movement continues to cite this kind of work as a foundation for its concern about dangers of vaccination. And so, there is a problem once a paper is in the literature and people believe it and they cite it and rely on it, and simply putting the word retracted over it, doesn't negate all of the impact that that work has had and the possible wasted effort that might be spent chasing blind alleys.
Chris - What about the flipside of this, which is that we assume by your reasoning, that all of the data in a paper is fraudulent? We take the case of Woo Suk Hwang, the Korean stem cell scientist, who famously had done all kinds of nefarious things with data falsification. Actually, some of his work was extremely good and absolutely sound. So, are we therefore, potentially, over-egging this pudding to a certain extent with the interpretations you're making.
Ferric - So, I think the concrete results of this study are that the actual dollar amounts that can be directly attributed to research that was fraudulent, are fairly modest, and they're not the major cost of research misconduct. I don't really think that this is the major cost. I think then we can move on to try to look at these costs of research misconduct that are much more difficult to put a number on but are much more serious. One of the things that we haven't really touched upon is what happens to the reputation of science? And I think this is of a great concern to scientists, because we only perform science because of public support for science and confidence that scientists are doing their best to create new knowledge that can be used for society's benefit. The damage done by people wasting time, going down the wrong direction, not knowing what's true anymore, I think, can be very substantial.
Chris - Ferric Fang from the University of Washington.
13:15 - The acid test
The acid test
with Kiersten Henderson, Fred Hutchinson Cancer Research Center
Why do some yeast cells grow old whereas others do not?
Dr Chris Smith spoke to Kiersten Henderson from the Fred Hutchinson Cancer Research Center about how aging occurs...
Chris - An interesting feature of many cell types is that they can divide asymmetrically, meaning that the mother and daughter cells are different, and the same can apply to aging with a progressively senescing mother cell repeatedly giving rise to youthful offspring, but how? Kiersten Henderson has been looking yeast to find out.
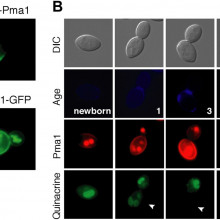
Kiersten - We're interested in understanding how cells age and we're doing that using a microbe, which is a model organism called budding yeast. And we simply began by asking what happens to cells as they age, and we did that by looking at the functions of different compartments or organelles inside the cell during aging, and we noticed that the function of a particular compartment, called the vacuole, declines with age. So, normally what happens in the vacuole, it stores ions and amino acids - so, things that could potentially be harmful to the cell but are necessary to cells, and also proteins are degraded there, they are unwanted. And we noticed that vacuole function declines pretty early in aging, and we wanted to figure out how it is that that occurs.
Chris - How did you do that? How were you investigating what those vacuoles were doing and why they deteriorate, or for want of a better phrase, "Clap out," as the organism ages? And what is aging in the context of a yeast?
Kiersten - Well, aging in a yeast is defined, and this happens to our cells, human cells also, they only undergo a certain number of divisions and then they stop dividing, and they take on characteristics of age. They just don't look as healthy. A really interesting feature, though, of budding yeast is that aging is asymmetric between the original cell and the daughter cell that it produces. We were able to use that observation to ask, because we expect that the factors that are causing aging will also be asymmetric between mother cells and their daughter cells. And we found, when we looked at the vacuole, yes, it's function declines during aging but the function is regained in daughter cells, so, they're born young even though the mother cell is aging. They're rejuvenated. So, we wanted to see what was it that was causing this decline in vacuole function but the rejuvenation of vacuole function in daughter cells. And we started by looking for proteins that were retained in mother cells during aging, so it just weren't passed on to daughter cells, and we found, one in particular, the protein at the membrane of cells and it's job is to regulate the acidity of cells.
Chris - It's fascinating to think that an aged mother can have a pristine cellular offspring, or daughter, isn't it? And it's interesting that the yeast knows in inverted comas "how to partition," so that you get a pristine daughter and an aged mother. What are the chemicals that are contributing to the aging, the ones you've discovered that are these membrane proteins? What do they actually do, and why are they partitioned asymmetrically in that way do you think?
Kiersten - Well, we don't know why they're partitioned asymmetrically, but right now, one of them in particular that we find initiates aging and affects the function of the vacuole. It's job is to regulate the acidity inside the cell, so it moves protons, which are acid equivalents outside of the cell and that protein that's on mother cells is very, very low on newborn daughter cells as they emerge from the mother cell as they are being formed. That actually allows the pH or the acidity inside the newborn daughter cell to be normal again. The amount of acidity in a mother cell is actually declining with age but it becomes normal again when daughter cells are born, and that actually allows for normal function of organelles inside, like the vacuole inside daughter cells.
Chris - Do you think this is cause or effect? In other words, are you seeing something which is a consequence of the aging process, or do you think this is something that's causal of the aging process?
Kiersten - Our goal was to identify factors that actually cause aging, so we're able to modulate the amounts of this particular protein at the membrane and actually affect lifespan. So, if we decreased the amount and make the inside of the cell more acidic and allow for a more normal function of organelles, like the vacuole, this actually extends lifespan.
Chris - So, why don't all cells then just do that themselves? Why don't they evolve to perpetuate themselves and therefore not age as fast or is that indeed, what does happen in say, cancer cells which have an indefinite lifespan potentially?
Kiersten - So, there's definitely a trade-off for this function. It's an essential function for young cells, so they have to be able to do this to grow properly, so they can't simply reduce function. So, there's definitely a trade-off because there needs to be less function to get a longer lifespan but it causes aging ultimately.
Chris - And of course, the killer question that everyone is dying for me to ask. Have you found the fountain of youth? Is there a way to reverse this process, or to minimise the aging process on the basis of what you found, at least, in yeasts that we could potentially extrapolate to our own cells and slow down aging?
Kiersten - Well, everyone who discusses my work with me likes to joke that proton pump inhibitors, which change the acidity inside cells, could potentially extend lifespan, but we really don't know yet how that would affect things.
Chris - So, maybe I'll put myself on Omeprazole, just in case. That was Kiersten Henderson from the Fred Hutchinson Cancer Research Institute.
19:11 - Keep it clean
Keep it clean
with Julie Simpson, Andrew Seeds, Janelia Farm Research Campus
A suppression hierarchy determines the order in which a fly cleans the different parts of its body.
Andrew - So, the idea was that if we were to cover the body of the fly in dust, the fly would be faced with a decision on which of those body parts to clean first. What we found was that they will immediately focus on cleaning parts of their head, particularly, their eyes and then they would move on to clean their antenna, and then at a certain point, they would groom the posterior parts of the body. So, over time, they have a higher probability of performing specific movements going from the head to the back of the body.
Chris - Julie, have you been able to find out the origin, neurologically, of these particular movements and how are you attacking that?
Julie - One of the wonderful things about working with fly is that we have genetic tools that allow us to drive expression of proteins of our choice in particular groups of cells in the nervous system. And each one of these stocks or lines of flies allows us to target a different discreet group of neurons in the nervous system of the fly. We use this collection of lines to determine which of those clusters disrupt or trigger aspects of grooming behaviour.
Chris - And when you do this, Andy, what do you see?
Andrew - Well, one of the big surprises when we activated these different neural subsets, we could activate very specific grooming movements. So, we found that the grooming behaviour could be almost divided up into its distinct component parts and when we've looked at the neurons that were involved in driving the specific movements, we found that they were sensory neurons and neurons within the brain that can drive very specific grooming movements.
Chris - Would you therefore say they're almost like a pattern generator, and that you activate that pattern generator and you get that particular pattern of grooming in that particular body part?
Andrew - That's correct.
Chris - So, Julie, does this mean then, that these pattern generators are connected together, and if you've got a sort of sequence that Andy's saying, you start with the head and work your way backwards on the fly. The one which is at the back is furthest down the pecking order and is turned on by the one before it, but suppressed by all the ones before that.
Julie - I would say that we're activating the behaviour upstream of the pattern generators, whose identity is still not known, and what's happening in our assay is that we're activating all of the triggers for the different behaviours and an upstream behaviour suppresses the ability to execute any of the downstream ones. When you put dust on the head, at the top of the hierarchy, they clean their heads and then they stop. That behaviour does not trigger any of the downstream behaviours.
Chris - So, with that observation in mind then, what do you think is going on? How do you account for that? If one thing's not triggering the next, how do you get that effect?
Andrew - So, if you go back to the original beginning experiment where we put dust on the whole body of the fly, what these essentially does, if you consider these different movements to be independently triggered, each body part has its own compliment of sense organs that can detect the dust. So, if you have dust on your eyes, in flies, they have bristles that can detect that dust on the eyes and then direct movements to the eye. The same goes for the wings and the abdomen; they all have their own independent way of sensing the dust. So, when you completely cover the body of the fly in dust, essentially what you're doing is making the flies want to groom all of their body parts, so in essence, the different movements are in competition with each other, so there is a hierarchy of suppression. The eyes, which are groomed first, that has the ability to suppress everything that follows it in the sequence. Whereas, something like he abdomen which comes after the eyes would not be able to suppress eye grooming, and it would have to essentially wait until eye grooming is completed. So, it's a competition, and it's almost as if the rules of the competition are pre-determined by some internal mechanisms that we have yet to figure out.
Chris - And if one thinks about animals like a cat which does have a very stereotypical way of grooming itself, so does members of the rodent family, mice and rats. Do you think you can use what you're seeing in the fly as a model for what we see in higher organism, and even in humans?
Julie - I think the idea that there are many different schema for how you produce behavioural sequences is a useful general insight. We look around and we see many different behavioural sequences, things like bird song, things like human language, could all be considered sequential organization, and there would be lots of different ways you might produce those sequences, but the way that we have found is quite different. All of the things are triggered at the same time, but one can suppress the other, and so, this suppression hierarchy results in sequential execution. This is an alternative way to make a sequence and may indeed turn up lots of behaviours, now that we know to look for it.
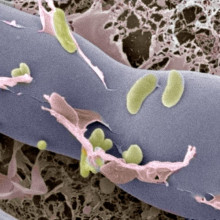
25:27 - Two against one
Two against one
with Christian Hertweck, Leibniz Institute for Natural Product Research and Infection Biology
Bacteria and fungal cells join forces to cause rice seedling blight.
Chris - Fungi are common plant pathogens, and they often use toxins to break open plant cells to liberate raw materials that they can scavenge for their own use, but the fungus doesn't necessarily have to make the toxin itself. Christian Hertweck has discovered that the rice blight fungus contains a bacterium that lives inside the fungus, and in return for board and lodging, produces a potent chemical weapon that the fungus uses to attack the plant.
Christian - For a long time, it has been known that a particular fungus can cause a plant disease named rice seedling blight. The rice seedlings swell and then they decay, that is because the toxin that is secreted by the fungus. The toxin is known to inhibit the cell division of the rice seedling.
Chris - Does the fungus make the toxin or does it get it from somewhere else?
Christian - Well, actually, that was a big surprise. When we wanted to find out how the fungus makes it, we found out that it is actually not producing these toxins itself but it harbours the bacteria that live within the cells of the fungus, and these bacteria have all what is needed to make the toxin.
Chris - So, this is a sort of symbiotic relationship. The bacteria live with fungus and they give rise to this toxin, which the fungus then deploys at its victim, the rice plant, presumably, so both can access nutrients by breaking down the plant cells.
Christian - Right. So, these bacteria belong to a genus called Burkholderia, and these bacteria are known to inhabit various habitats, so they have already been known to live in symbiosis with various organisms but not with fungi so much.
Chris - Two questions are rising then, I mean, one, how did this happen? and two, how did the bacteria form this alliance with the fungus? How did they get recruited? How did they get in? and then how do they get deployed into making this toxin?
Christian - In fact, it is a surprise that the bacteria can live within the fungal cell, and of course, the question is how has it ever got inside? We now recently found out how it does it? So, because that's not a trivial thing, the fungus is covered by a thick cell wall, which is typically a barrier to everything that comes from outside, so the bacteria found a way to dissolve a particular spot in this barrier. So, just enough to slip inside.
Chris - How did you do this? Did you literally watch fungal hyphae, the little protrusions under a microscope, so you could see the bugs getting in and out?
Christian - It's actually not so easy to see them. In fact, it has been overlooked for decades. So, we were quite surprised to first detect the bacteria inside by a technique that is called, confocal laser scanning microscopy, and using this technique, we could first, localize the bacteria within the fungal hyphae, but the question of course was how can we catch these bacteria in action? and that was just recently achieved, taking some snap shots of the bacteria on the way inside the fungal cell.
Chris - You have these microbes. They have the ability to produce a cell wall dissolving chemical that gets them into the fungus. How does the hole get healed afterward, and how do the bugs get out again to then spread and penetrate other fungi or multiply?
Christian - In fact, we don't yet know how the hole is closed again, but we know that the enzyme that is secreted to dissolve the cell wall is actually only produced in a small quantity. So, that probably means that only where the bacterium meets the fungus, the concentration may be high enough to just to make a small hole where the bacteria can slip through. This symbiosis persists by just cracking the spores, and without the bacteria, this particular fungus cannot sporulate anymore. So, the trick is, that in the presence of the bacteria, spores are formed and in each spore, you can find the bacterium. So, when a spore lands somewhere and the fungus germinates, the bacteria are already present.
Chris - How do you think this interaction got started? In the first place, if you look at the relationship and you ask those sorts of questions, genetically, how do you think this happened?
Christian - A plausible scenario would be that initially, these bacteria took fungi as a prey, and typically, fungi are also sensitive to the toxin that is produced by the bacteria but the fungi that were now chosen as a host, they are resistant to the toxin. So, what we think is, that there has been a so-called, parasitism-mutualism shift. That means, initially, the bacteria was an enemy of the fungus but then it has become something like a friend, or that's at least what we think that it is now because they don't harm each other.









Comments
Add a comment