The cocktail party effect and how the brain decides which sounds to attend to, genes dismissed as dead relics turn out to play significant roles in inflammation, iPS cells reproduce degenerative retinal disease, the genetic responses to flu jabs, and the discovery of stem cells in schistosomes...
In this episode
00:44 - The cocktail party effect
The cocktail party effect
with Sundeep Teki
Chris - Hello. Welcome to the eLife podcast episode number 3, produced by the Naked Scientists. I'm Chris Smith. In this edition, how skin cells were turned into retinal cells to discover the cause of a patient's blindness, improving vaccines by studying the genes that are switched on when a student gets a flu jab, and the stem cells that might be the Achilles heel for one of the worst human parasites. First though, to the question of how our system of hearing, despite being constantly assailed by a barrage of sounds from a range of different sources can pay attention just to those sounds that are meaningful - for instance, just one person's voice in a crowded, noisy room. Sundeep Teki from UCL has discovered that the brain is highly tuned to spot when certain frequencies start and stop at the same time. This is how it knows which ones to listen to.
Sundeep - In a realistic environment, we have lots of sounds which have overlapping frequencies, the start and stop at different times. So, what we try to do in our study so far is to come up with a signal which more accurately represents what we really hear every day in our life. So, instead of our previous work which was just based on two frequencies which were alternating in time, we came up with a more complicated stimulus which has several frequencies in it.
Chris - I've got a sample of that, so we could just have a listen to this (sound recording)...
Sundeep - In a way, it's a bit like noise. So, what we did within this sound was to make a few frequencies, start and stop at the same time and what happens is that that particular segment of sound kind of pops out against the background.
Chris - Well, let's have a go. (sound recording). And there's a very obvious stand out difference, isn't there?
Sundeep - Exactly, so there is this pop up effect and listeners can get it quite quickly. We didn't have to train them too hard in order to get that.
Chris - Well, what does this show? Why is this important?
Sundeep - Right. So, the pop up effect suggests that the brain has some kind of a mechanism which is able to extract this pattern that we've imposed based on a few frequencies that start and stop at the same time. So, we think the brain is sensitive to these correlations and it tracks the time on the signal. And if certain frequencies start and stop at the same time, it registered that there was something here and it might lead us to suggest that these sounds might belong to one particular object. So, we know that in this background of multiple sounds, we heard something salient which popped out.
Chris - Does this mean then when I'm having a conversation with someone and they're speaking to me, that I am at the same time is listening to what they're saying, I am also running ahead in the conversation, almost predicting what I think they're going to say and then my brain is listening out for that predicted regularity of pattern, to then extract that information from the sound?
Sundeep - Indeed. Our voice is very rich signal, not only frequency and time information, but also information about your vocal tract. So, after a few seconds, I think the brain kind of forms a template of your voice and it's building up with more and more information. The model prediction becomes better and better.
Chris - So, when you've got your subjects in the laboratory and you're playing them these sounds, what sorts of measurements are you making? How are you testing the person's reaction to those sounds and the differences?
Sundeep - So, what we did is, we invite listeners to a sound proof booth. Sounds are played which have different properties. So, we varied two parameters, the number of frequencies which became synchronous and the time for which it became synchronous. And the task of the listener was who wants to press a button as soon as they heard something pop out. So in each block, half of the sounds had a target in them and half of the sounds did not have a target in them. And basically, what we found is that they are extremely good at detecting these targets even with very little practice. And the performance improves if the number of frequencies that are synchronous are more and more, and the time for which they are synchronous are more, suggesting that the brain is kind of integrating information from both the frequency and the time domain to be able to detect the target.
Chris - So, armed with those observations, what are you concluding and what will be the logical next step?
Sundeep - Apart from doing this kind of behavioural testing, we also did a modelling analysis in our study. The main hypothesis of this model is that the brain is indeed looking at the time information and trying to calculate correlations between different frequencies. So, if there is a strong correlation in time for certain frequencies, then it might belong to one object and they might stand out from the rest of the background. Our data which is based on a signal which is very realistic supports this model which is known as the tempo-coherence model. We think that this is the way forward.
Chris - Because practically speaking, where we would really like to go with this apart from understanding how we work is to endow our computer with the same abilities that we have because I'm sure everyone who's ever spoken to a computer down a telephone and tried to tell it what you want, the problem is obvious.
Sundeep - Yeah, I know what you're talking about. So, speech recognition is still very intense topic of research. Results from fundamental neuroscience studies like this one may help inform the engineers to come up with more sophisticated algorithms that they can implement in their devices. So hopefully, we hope that this might lead to some kind of a practical benefit in hearing aid design or machine listening devices, and so on.
Chris - Sundeep Teki. He's at the Wellcome Trust Neuroimaging Centre at UCL.
07:01 - Turning on pseudogenes
Turning on pseudogenes
with Howard Chang
Chris - Within the human genome are large numbers of what are called pseudogenes. These closely resemble real genes that actually make proteins. But they've been written off as derelict DNA sequences that have lost their original functions and are no longer needed. But this is not so says, Stanford's Howard Chang who's found that certain stimuli turn these so-called dead genes back on. And they appear to have a major role to play as he explained to Kat Arney.
Howard - So, we were interested in the gene expression programme, just the set of genes that will react when cells are exposed during inflammatory stimulus. So, inflammation is a very sort of common, universal response of tissue to injury or infection. That's when if you hit your knee then it gets swollen and red, that's inflammation. And so, we wanted to understand what kind of genes would turn on or turn off when cells are exposed to signals related to inflammation.
Kat - And so, how did you go about looking at that? What sort of cells were you using and what techniques did you try?
Howard - Using the kind of cell type called fibroblast, they're kind of what makes up what is called the connective tissue. We use the method that can basically count the different kinds of RNA molecules that a cell would make. And so, every RNA molecule was sequenced.
Kat - And an RNA, that's like the message that's made when a gene is switched on?
Howard - That's right. So, the genetic information in this DNA is turned into RNA, when the cell wants to use a particular bit of information. And so, by looking at which RNA molecules are made, you can tell which genes are being turned on.
Kat - What did you find?
Howard - Well, what's surprising was that in addition to the usual large number of protein coding genes, so-called messenger RNAs, there were a large number of RNAs that were not coding for proteins. These are called long, non-coding RNAs. So, this is a class of newly recognised genes which is actually fairly large. So, there are probably over 10,000 of these long non-coding RNAs in the human genome.
Kat - So, these are messages that are written out from the DNA, but they don't tell it to make a protein. They just do something else.
Howard - That's right. Only a very small number of these RNAs have really been studied. And so, there's a lot of questions about, really, what are full set of long non-coding RNAs and when do they even come on, what kind of signals they respond to, and what they might do. So, one part of the work was to characterise these long non-coding RNAs that will respond to inflammatory signal. And a further surprise was that we noticed that there was a set of these long RNAs that come from the so-called pseudogenes. So, these are genes, are thought to be basically dead genes. Genes found their ways to becoming molecular fossils if you will.
Kat - That's really weird because it was thought that these pseudogenes did nothing at all.
Howard - That's right. So first of all, I was so surprised and so, we looked more carefully at a particular pseudogene and also, some of the other long non-coding RNAs that were turned on in response an inflammatory signal. And the further surprise was that now, with the pseudogene turned on in response to let's say a signal, but the response was exquisitely specific. A certain kind of inflammatory signal let's say for bacteria will turn on the pseudogene. Therefore, the set of long non-coding RNAs including pseudogenes was so specific that they were like an internal reflection of what the cell was seeing on the outside. You could tell what the cell was being bombarded with, what kind of infectious or inflammatory signals are coming in by looking at the pattern of these non-coding transcripts.
Kat - And there was one in particular you studied. Tell me a bit about that one.
Howard - This is a pseudogene RNA that we focus on because first, it came out in our screen that it was induced by inflammatory signal and there are other qualities that are interesting in these RNA was made but it was almost entirely in the nucleus, it didn't go into the cytoplasm. And then we noticed that this RNA actually have a role in controlling the inflammatory response. It turns out that those RNA, when it comes on, the pseudogene RNA is actually a part of the process of damping inflammatory response back down. It's part of the normal feedback to kind of shut the whole response down again. We call this RNA Lethe after the Greek 'river of forgetfulness'. So, they underworld analogy is because of pseudogenes being essentially dead genes. So, we're both from the idea of the underworld. But there's also this idea that, let's say, the cell encounter some sort of inflammatory response or some sort of infection and it's gone through this battle and fighting it off. And now, it's time to go back to your normal life, to move on new life and you had to forget about this inflammation. And so, you have to turn the whole response down and Lethe is part of that process. So basically, in the absence of Lethe, the cell will basically keep going forward with the inflammatory response. It doesn't forget even if the battle is over.
Kat - Do you think that there are other pseudogenes lurking the genome that have this kind of roles that are actually not drunk and not dead, and could be very active and important?
Howard - Yes, indeed. So, I think over the last several years, the other investigators have found have resembled from the ENCODE project that many pseudogenes are actually being transcribed, that they're made. They're evidence of their activation and that work was led by a Professor Mark Gerstein from Yale. More recently, other people have realised that pseudogenes because they're copies of normal genes, they have many of the same regulatory sequences embedded in them. And so, when the pseudogenes are made, because of these regulatory sequences, compete for different cellular factors.
Kat - Where do you go next for this research? How are you taking it forward?
Howard - Well, so a very interesting and unexpected was that we found that the pseudogene regulation actually changes organismal age. And so, some prior work we had done has shown that as organisms get older, some of the gene regulatory systems involved in inflammation are just activated and stays on over time. And we didn't know why that was the case. So, it turns out that the pseudogene, Lethe, that those activated Lethe actually decreases with organismal age. So somehow, this negative feedback system is gradually lost where organisms get older. So, this is a very tantalising clue for how, perhaps, the whole system of inflammation and genes regulation aging could break down. And now, there's a surprising clue that this might actually have to do with pseudogenes.
Chris - Stanford's Howard Chang. He was speaking with Kat Arney.
14:01 - Looking into blindness
Looking into blindness
with Budd Tucker
Chris - If you have a patient with an inherited form of blindness and you want to know what gene is causing the condition and how it contributes to the disease process, wouldn't it be nice if you could turn some skin cells into some of the affected retinal photoreceptors in order to be able to unpick the pathology?
Budd - My name is Budd Tucker. I'm an Assistant professor at the University of Iowa in Iowa City. Retinitis pigmentosa is one of the many forms of an inherited retinal degenerative disease. So, patients with retinitis pigmentosa present with reduced vision. They will often have poor night vision from very early in life. As the disease progresses, there is cell death in the outer retina and these cells are the light-sensitive photoreceptor cells. So, once those cells die being a part of the central nervous system then you essentially go blind. And when there's a complete loss of those cells then there's no vision observed at all. So, the question is, how do we treat someone with retinitis pigmentosa or retinal degenerative diseases like this? There is good evidence now that transfer of genes, so, wild type full length genes can be used to restore vision or prevent vision loss in people with genetic disease. So, in order to do that, you would need to know what the disease causing genes are and in turn, you would need to know what the disease causing mutations are and how that disease would normally progress.
Chris - So, do we not know what the mutation is that is causing retinitis pigmentosa cases?
Budd - See that's the exact problem we're talking about because retinitis pigmentosa currently can be caused by as many as 60 different genes and hundreds of mutations in those genes. That's what we've currently identified. Well, a year and a half ago, we identified a completely novel retinitis pigmentosa gene. We believe that because of the cases that are still out there with unidentified genes and we know they have RP or retinitis pigmentosa, that there's going to be in total probably 100 different genes which were responsible for causing this disease.
Chris - Is the issue then not just one of genetic sequencing? We know the person appears to have the disease. We take some DNA from them, we sequence their genome and we identify where in their RP gene things have gone wrong.
Budd - Right, so that is the issue. Why does that not work? Because for this patient, we knew he had retinitis pigmentosa, but we could not find his disease-causing gene. We could not find the mutations in his disease-causing gene. So, one of two things were true. He either had mutations in a disease-causing gene that we already knew. We just couldn't find those mutations because they were in non-coding space or this was an entirely new gene that we needed yet to find.
Chris - So, how did you approach that?
Budd - So, the way we approach that, we did rounds and rounds of exome sequencing, just as you said. So, we sequence this entire individual's whole coding region, three to four times. We could not find two disease-causing mutations in any retinitis pigmentosa genes. So, the way we thought to approach it then was to start with the person's retina. Well, how do we do that? It's very difficult to get a retinal biopsy from someone. It's like getting a brain biopsy. It's part of your central nervous system. So, the goal would be to make retina from this individual. Then what we could do is look at the transcript in the retina. So, is the transcript and in turn protein expressed.
Chris - So you're saying, you'd take some other cell source in the body and reprogramme that cell source to recapitulate the retina and then you can quiz that tissue and ask, what is going wrong in here to generate this RP phenotype in this person.
Budd - Right, precisely. We do that from taking a biopsy from the skin and we reprogramme that using four transcription factors back into stem cells called induced pluripotent stem cells or iPS cells and then we used a protocol that we have developed to take these cells and drive them into a photoreceptor or retinal and then photoreceptor lineage.
Chris - So, you turn these cells into the sorts of cells that are affected by the disease in the retina by effectively mimicking the environment that they would grow up in so they turn into that particular cell type. How do you then ask, why in this individual, they get the disease?
Budd - So, what we did in this case is we took the transcript, isolated RNA and then we did simple PCR up and down some of the suspected genes. When we did that, we found one mutation that we had already identified in whole exome sequencing in the DNA which was just a single point mutation. Then we found another mutation which actually was in a non-coding region, which causes single base pair switch which was in a cryptic splice site. So then what happened was that a large piece of the non-coding DNA got stuck in-between coding regions and started to code. So, that caused an insertion of a stop-coder.
Chris - Do you know why that abnormal protein triggers the disease in this person?
Budd - We think we do. So, this gene usherin encodes for a protein called usherin which is a very large extracellular matrix protein. Now, this very large extracellular matrix protein is tethered to the photoreceptor. It's actually expressed at the base of the connecting cilium of the photoreceptor. So, the photoreceptor has a bridge or connecting cilium which is vital, which connects the inner portion of the photoreceptor to the outer portion. The outer portion is the structure that really holds the photo pigments and things like this - the machinery required for detection of light. Yet, all of those components for the most part are made in the cell body. So, they need to be trafficked across this bridge. Usherin is responsible for something within that structure. We're not quite sure exactly what it is at this point. We do know what the protein, where the protein is expressed. Interestingly, when you transplant this individual cell into a mouse model - a retinal disease, this individual - I mean, these human photoreceptor cells go on to develop normal looking photoreceptor cells. They make outer segment like processes and they have normal - what's looks like normal - connection cilium. So, it doesn't appear to be stimulating an early developmental defect in photoreceptor development in this individual. Clinically, this individual did not lose his vision or start to lose significant vision until he's mid to late 40s. So, it seems like what's happening here was that the mutation in this large gene is over time inducing stress of the endoplasmic reticulum, so, something known as ER stress. So, it suggests that this mutation is causing a protein misfolding response, gums up the endoplasmic reticulum and over time, the cells succumb to this ER stress and die.
Chris - Obvious question, but did you go back to the original patient, armed with your mutations you've found and check that they were actually what was wrong with the patient. They weren't just mutations that cropped in the reprogramming process to make those stem cells into retinal cells.
Budd - Absolutely. So, we went back and we sequence through this gene, this individual gene from his blood and we found that mutation in his gene.
21:25 - How genes influence our response to the flu vaccine
How genes influence our response to the flu vaccine
with John Belmont
Chris - Vaccines are regarded as one of the most important advances in preventative medicine. But they don't always work. Why is this and can the patterns of gene activity, this ensue when a person is vaccinated give us clues as to why and how to improve things? John Belmont...
John - Influenza vaccines are incompletely effective. Influenza is an extremely important disease. Many millions of infections occur every year. In fact, we think that about 2% to 7% of the entire population gets an influenza infection every single year. The mainstay of preventing influenza in the medical complications is seasonal vaccine. But because the vaccine doesn't protect everyone, what we wanted to do is try to use new genetic methods to try to get a better understanding of what were the differences between people making a higher response and those making a lower response.
Chris - How did you do that?
John - We carried out two clinical trials. These are medical experiments, so we went to the Texas A&M University about 100 miles from Houston and we enrolled college students by and large. This is a really interesting group of subjects because they were very healthy and many of them exercising and not having a lot of other kind of medical issues that complicate our interpretations. So, we then immunised these young adults with seasonal influenza vaccine and tested them both before they got the vaccine and at the three timepoints - 1 day, 3 days, and 14 days after the vaccine was administered.
Chris - What specifically were you measuring?
John - We did something that had not been done before and that is, we were able to measure all of their gene activity in a global way. So, we used an inexpensive microarray technology that allowed us to get an estimate of the activity of all of the 21,000 or so genes that possibly could be expressed.
Chris - This is on blood?
John - That's right, but the distinction here is that we also extracted RNA so that we could measure the gene activity. At the same time though, we did extract the DNA because what we wanted to do was to find the common variance that all of us have to see what impact those common genetic variance had on the level of the gene activity. Essentially, what we're trying to do is build a bridge between the very complicated final outcome that is making the antibodies in response of the vaccine and the intermediate changes in gene activity.
Chris - What did you see in the blood of these participants at those different timepoints after the vaccine?
John - There's a very characteristic 3-phases of the response. First is the baseline and then in the first 24 hours, there's a response of the innate immune system. These are the basic responses that the body has to any kind of infection, a virus or a bacteria. And then there is a kind of revolution that indicates that the blood cells are proliferating and what is really interesting about this is that we have an independent data set that's not described in this paper that gives us an indication of what happens during a naturally occurring influenza infection. And the vaccine response is very parallel to that - it's just the vaccine is like a weak version of what happens during a natural influenza infection.
Chris - Is this a killed vaccine that you gave them?
John - Yes, that's right. so, the vaccine itself is made from killed virus particles.
Chris - How did you then establish downstream vaccination who had responded and who hadn't?
John - Well, we spent a lot of effort making mathematical models of that response, but we did actually see a fairly clear pattern, that is, there were clearly some high responders and some that were lower than we expected. And so, there is an understandable pattern in the distribution of responses.
Chris - So, is the idea here that you could then say, well, can we find why those people who don't respond so well appear not to and then tweak the vaccine in some way, in order to beef up the response in those people who are poor responders.
John - That's clearly what we're hoping for in the long run, by getting a better understanding of what mechanisms are quantitatively different between people who are making a high response. And those making a low response, we would hope to be able to adjust either the vaccine dose or some of the qualitative aspects of how the vaccine is prepared, to try to overcome those who are making a lower response. So, I mentioned before that the vaccine is stimulating pathways that are a lot like what's happening during a naturally occurring infection. So, if we try to make a vaccine that actually doesn't stimulate those pathways, it's likely not to work as well. And so, incorporating components into the vaccine that stimulate the innate immune system may be quiet important in having an effective vaccine.
Chris - Now, one thing that's a major problem for certain people is that they're non-responders to a range of vaccines. Hepatitis B is a good example, especially for healthcare workers. So, could you take a technique you've identified here for flu and apply this to try to work out why people don't respond to other kinds of vaccines?
John - We're actually hoping that our study design approach will be a very good way to test vaccines early in their development, so that we could better understand when a vaccine is going to be effective and try to make adjustments in manufacturing. So, the answer is, we certainly hope so and we would like to work with other groups involved in developing vaccines.
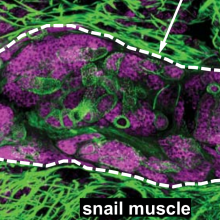
27:27 - Stem cells and tropical diseases
Stem cells and tropical diseases
with Phil Newmark
Chris - And finally this month, human worms. Phil Newmark and his colleague Bo Wang from the University of Illinois at Urbana-Champaign have discovered a population of stem cells in schistosomiasis bilharzia that enable the worms to repair themselves when the human immune system attacks, and to enable the parasite to massively increase its numbers by dividing asexually in aquatic snails. As such, they represent a major therapeutic target.
Phil - In this study, we were interested in asking how it is that these really important human parasites called schistosomes generate so many infectious stages of their life cycle. Many parasites wind up using both, what they call a definitive host in which they can reproduce sexually, as well as an intermediate host that the life cycle also has to go through. And so, these organisms have to be able to infect a snail. What happens in the snail is something truly remarkable in that, a single creature that hatches out of an egg that comes from an infected human for example can enter a snail and produce thousands and thousands of the stage of the life cycle that can now swim through the water and infect humans.
Chris - Why is that remarkable Phil, that something could produce thousands of infectious progeny like that?
Phil - Well, it's interesting because basically, what you have is, inside the snail is one hatched creature, will go through an amazing asexual reproduction. And so, we were interested in this asexual reproduction because the major focuses of my laboratory is to study regeneration in planarians which are a related flatworm. These planarians are famous for their ability to regenerate. So, a small piece of the worm can regrow an entire new animal. Part of their regenerative abilities leaves these animals to also be able to reproduce asexually by fisioning. So, what we were intrigued by was this asexual reproduction in the snail which appears to be driven by a similar kind of stem cell we see in planarians. We were interested in knowing how this bag of stem cells when it enters the snail can now basically produce thousands and thousands of embryos without fertilisation. One of the things that inspired us to start looking at this was, we published a paper earlier this year showing that the adult parasites, the adult schistosomes that reside in a human host have stem cells that are very, very similar to those that we see inside the adult planarians.
Chris - What do the adult worms, the schistosomes in the human doing with those stem cells because they don't presumably don't serve much of the same process of the human because once it's in the human, it's then more interested in shedding eggs, isn't it?
Phil - Yes, so what we think they're doing in the human is, these adult schistosomes can survive inside a human host for decades, during the course of that long lifespan in hospitable environment of the human bloodstream, they must be experiencing tissue damage, and they must have to replace cells that are just lost during the course of wear and tear. And so, we think that the stem cells in the adult are serving the purpose of maintaining all of the tissues over the animal's long lifespan.
Chris - So, how did you try and investigate further what these stem cells were doing in the snails?
Phil - The first thing that Bo did, he found an RNA binding dye that could label these cells very nicely. Bo was then able to measure when those cells begin to divide as the creature transitions from the outside of the snail to the inside of the snail, part of the lifecycle so he could measure when those cells begin proliferating. He saw that over the first couple of days of this transition, there was a dramatic change in the number of these cells and how much they were dividing. And so, what he then did was to use next generation sequencing technology so he could basically just isolate RNA from the stage before it's in the snail, and then from the stage after it's been in the snail for several days. And there has been time for these cells to really proliferate and expand, we should find genes that are expressed in these stem cells. And then he looks at what those genes are, he sees that they share very many similarities with the kinds of genes we see in the adult schistosome stem cells and in planarian neoblasts.
Chris - Effectively, what we've got here is a population of stem cell-like cells that are present in the organism when it infects a snail. This enables the organism to dramatically increase the population because it's clonally expanding inside the snail, coming out into the water so it can then hit many, many people. But then those same stem cells endow the adult worm with a defence capability and regenerative capability against human immune attack.
Phil - That's kind of how we're thinking about it. We've seen these intriguing similarities. And now, what we really would like to do is to ask exactly how these cells are propagated throughout the whole lifecycle.
Chris - And thinking of therapeutic potential because one always has to consider - I mean, schistosome is second only to malaria probably as one of the world's worst parasitic infections and certainly the biggest disease burden. So, if you've got this population of cells that are crucial to the behaviour of two of the key components in that lifecycle and you can single them out, do you think there's a therapeutic avenue there?
Phil - Yes, that's exactly right. so, we think long term as we study these cells more and learn more about what they're doing in the context of the adult, as well as how they're required for generating the infectious portion of the lifecycle, that yes, these could be really important targets for compromising the viability of the adult worm or perhaps, preventing their propagation. One of the intriguing things is, if these cells are very similar, maybe we can use the snail stages, the lifecycle as a way to screen for drugs that will inhibit the ability of these cells to function that will also work in the adult.









Comments
Add a comment