Post by: thebrain13 on 24/09/2012 22:42:57
Post by: damocles on 25/09/2012 00:04:58
Post by: thebrain13 on 25/09/2012 01:22:47
Post by: CliffordK on 25/09/2012 01:55:38
that has 3 neutrons. Is it possible for helium-1 exist? and if not why not?Not exactly.
Helium, of course, by definition has exactly 2 protons, and can vary with the number of neutrons.
Here is a list of the helium isotopes. (http://en.wikipedia.org/wiki/Isotopes_of_helium)
Helium-4 is (
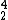 He) is the most common form of helium on Earth since it is a nuclear decay product. It has 2 neutrons and 2 protons for a molecular weight of 4.
He) is the most common form of helium on Earth since it is a nuclear decay product. It has 2 neutrons and 2 protons for a molecular weight of 4.Helium-3 (
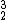 He) is also stable, it has 1 neutron, and 2 protons for a molecular weight of 3.
He) is also stable, it has 1 neutron, and 2 protons for a molecular weight of 3.Helium-2 (diprotium), (
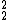 He) has no neutrons, and 2 protons. It is very unstable, and decays almost immediately to deuterium, or Hydrogen-2 (
He) has no neutrons, and 2 protons. It is very unstable, and decays almost immediately to deuterium, or Hydrogen-2 (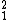 H).
H).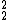 He →
He → 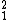 H + e+ + νe + Energy, where e+ is a positron (antimatter), and νe is an electron neutrino (http://en.wikipedia.org/wiki/Electron_neutrino).
H + e+ + νe + Energy, where e+ is a positron (antimatter), and νe is an electron neutrino (http://en.wikipedia.org/wiki/Electron_neutrino).By definition, one can not have a Helium-1 because that would mean 2 protons and (-1) neutrons for a molecular weight of 1.
Helium with more neutrons is unstable, with a half-life of less than a second.
Helium-5 (
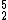 He) would have 2 protons, 3 neutrons, and decays with a half-life of about 700 ×10−24 s
He) would have 2 protons, 3 neutrons, and decays with a half-life of about 700 ×10−24 s
Post by: thebrain13 on 26/09/2012 23:35:04
Post by: Soul Surfer on 27/09/2012 15:29:25
I say approximate because many elements are mixtures of isotopes and do not have atomic weights close to whole numbers.
There are also other reasons why atomic weights are not exact whole numbers. The atomic mass unit is defined as one twelfth of the mass of a carbon atom (six portions six neutrons and six electrons) but the neutron is slightly heavier than the proton because of its lower binding energy. and most atoms have more neutrons than protons. You also have to correct for the binding energy of the atom which has a small effect.
Post by: evan_au on 30/09/2012 11:56:02
- For stable light atoms, the number of protons & neutrons is approximately equal.
- For stable heavy atoms, the number of neutrons exceeds the number of protons. You can think of the electrostatic repulsion between the protons, which tends to disrupt the nucleus; this is counteracted by the strong nuclear force from both protons & neutrons which tends to hold the nucleus together.
If there are "too many" protons to be stable, a proton tends to turn into a neutron (emitting a positron=β+ or swallowing an inner electron).
If there are "too few" protons to be stable, a neutron tends to turn into a proton (emitting an electron=β-).
...But other decay modes exist, like emitting a proton or neutron; heavy atoms can also decay by emitting an alpha particle or fission (splitting in halves).