Post by: Justin_Is_Here on 11/12/2014 01:22:48
Post by: Artyom on 29/12/2014 15:20:46
Maybe, it will work:
1) In a solution there are
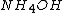 ,
, 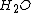 and
and 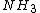 (
(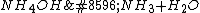 ). If you'd can remove impurities...
). If you'd can remove impurities...2) Then
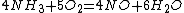
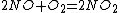
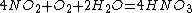
3) And then you can make all nitrate salts from
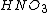 .
.P.S. But I'm not a chemist.
Post by: chiralSPO on 29/12/2014 15:47:36
Post by: lightarrow on 30/12/2014 16:36:00
Any aqueous oxidations of ammonia or ammonium salts I have come across lead to formation of nitrogen gas, not nitrate...Then Bacteria have better laboratories:
http://en.wikipedia.org/wiki/Nitrification
[:)]
--
lightarrow
Post by: chiralSPO on 30/12/2014 19:34:47