Post by: PmbPhy on 12/09/2014 18:29:08
Quote
Can an electron be in two places at the same time?poised a great number of times and in almost all cases the responder says "yes" where in fact the answer is "no." I'm looking for the source of such assertions because I want to write a paper on the subject. Can someone find the source of this question and the answer as well? Thanks.
Post by: evan_au on 12/09/2014 23:41:00
See: http://en.wikipedia.org/wiki/One-electron_universe
However, the question seems to be more aimed at the double-slit experiment (http://en.wikipedia.org/wiki/Double-slit_experiment) conducted with electrons, where the interference fringes suggest that a single electron has passed through both slits at the same time before hitting a target.
This is just like laser light, which can pass through two slits at once, causing an interference pattern - even if the light intensity is so attenuated that only one photon is in transit at once.
Post by: chiralSPO on 13/09/2014 00:02:15
Post by: lightarrow on 13/09/2014 11:10:02
Defined as the wavefunction which represents it, the answer is yes; defined as the properties of its detection the answer is no.
Defined as "an irreeducible representation of an Hilbert space on the Poincarè Group with charge -e, mass 9.11*10-31 kg and spin ħ/2" I would have some difficulty to answer.
--
lightarrow
Post by: JohnDuffield on 13/09/2014 16:22:58
Can someone find the source of this question and the answer as well? Thanks.I can't find the source, but the answer is yes. The electron has a wave nature. A wave is not some point-particle thing. It's quantum field theory, and the electron's field is what it is. It's always in more than one place at once.
Post by: PmbPhy on 13/09/2014 20:30:27
Can someone find the source of this question and the answer as well? Thanks.I can't find the source, but the answer is yes. The electron has a wave nature. A wave is not some point-particle thing. It's quantum field theory, and the electron's field is what it is. It's always in more than one place at once.
As is the case too many times John, you're wrong. It most certainly is not the case. As I said in the OP in fact the answer is "no." so if you have another opinion I have no interest in hearing it.
I wrote about this to a physicist I know. He got his PhD at Princeton and worked as a physics professor at Harvard.
Quote
The statement is nonsense. It arises from the mistaken idea that an electron is a little spinning object (made out of ...?). An electron is much more subtle than that.
A similar confusion exists with respect to the photon. While teaching the course from which my book Newton to Einstein emerged, I coined the sentence, "Light travels as a wave but departs and arrives as a particle." No classical concept--electromagnetic wave or tiny solid particle--corresponds to the truly novel nature of light.
Similarly, no classical concept can capture all the attributes of what we call "an electron."
My friend at Boston University, whose also a world renown physicist, also confirmed this for me too (since I always knew it but enjoy having things confirmed before I talking about them in forums).
Post by: jeffreyH on 13/09/2014 22:35:14
Post by: PmbPhy on 14/09/2014 07:26:35
Quote from: jeffreyH
I agree with you Pete but out of interest how does this relate to the double slit experiment. Is it a wave disturbance in the electromagnetic field that causes interference? Like an echo?Thanks buddy! :) The particles have a specific energy and that energy is used to determined the de Broglie wavelength. Then we have particles moving along different trajectories and as such they have a different phase when they get to the screen. That difference in phase causes an interference pattern to form.
Post by: JohnDuffield on 14/09/2014 10:54:44
As is the case too many times John, you're wrong. It most certainly is not the case. As I said in the OP in fact the answer is "no." so if you have another opinion I have no interest in hearing it.I'm not wrong about this. Your friend must have misunderstood what you were asking. The electron is not some little point-particle thing. We make electrons (and positrons) out of light waves in pair production (http://en.wikipedia.org/wiki/Pair_production). We can diffract electrons (http://en.wikipedia.org/wiki/Electron_diffraction). In atomic orbitals (http://en.wikipedia.org/wiki/Atomic_orbital) electrons "exist as standing waves". The electron has a magnetic dipole moment (http://en.wikipedia.org/wiki/Electron_magnetic_dipole_moment), and the Einstein-de Haas effect (http://en.wikipedia.org/wiki/Einstein%E2%80%93de_Haas_effect) demonstrates that "spin angular momentum is indeed of the same nature as the angular momentum of rotating bodies as conceived in classical mechanics." And when we annihilate (http://en.wikipedia.org/wiki/Electron%E2%80%93positron_annihilation) the electron with a positron, we typically get two light waves. The evidence is rock-solid, the wave nature of matter is not in doubt. And waves are not point-particles. Again, the electron is not some point particle thing that has a field, its field is what it is. And that field is always in two places at the same time.
I rather fear your friend gave you the brush-off with this. You should contact him referring to the above evidence, and perhaps he'll give you a considered response.
The particles have a specific energy and that energy is used to determined the de Broglie wavelength. Then we have particles moving along different trajectories and as such they have a different phase when they get to the screen...They have a wavelength and a phase because they are waves.
Post by: PmbPhy on 14/09/2014 11:50:48
I agree with you Pete but out of interest how does this relate to the double slit experiment. Is it a wave disturbance in the electromagnetic field that causes interference? Like an echo?I see from John's misunderstanding how you might have also misunderstood something too.
I think you confused a moving electron's electric and magnetic field as being an electromagnetic field that causes interference. That's not the case at all.
What electromagnetic field are you talking about? Is it the EM field of electron? No. It's the wavelength and phase of the wave function of the electrons.
As I said earlier. The frequency is due to the wave function. The electric and magnetic field of a moving electron doesn't have a frequency to it. However the location of the slits are related to the phase of particles as I recall.
Post by: JohnDuffield on 14/09/2014 13:19:43
Post by: yor_on on 16/09/2014 14:33:25
"To demonstrate the quality and value of the triplets, researchers tested local realism—finding evidence that, as quantum theory predicts, entangled particles do not have specific values before being measured.* Researchers also measured one of each of a succession of triplets to show they could herald or announce the presence of the remaining entangled pairs. An on-demand system like this would be useful in quantum repeaters, which could extend the range of quantum communications systems, or sharing of secret data encryption keys." http://phys.org/news/2014-09-charm-nist-detectors-reveal-entangled.html#ajTabs
It's just a example of the principle I think is there.
==
Also I had some papers defining the possibility of finding electrons existing in different places 'simultaneously', and experimentally so, but it's been some time since? I know I linked them here though, but maybe a year or two ago?
Post by: yor_on on 16/09/2014 14:59:20
=
You can do the interference pattern using just one 'photon' at a time, and the only way that singular 'photon' can create that 'wave pattern', as I know? is for it to be assumed to somehow interfere with itself, passing both slits simultaneously. Alternatively define it as a product of a field, observed. There is no real problem to this if we define under a arrow though. Under a arrow you should only find one outcome at a time, measuring. At least I think it should be that way, although it might also depend on how you define that outcome, aka setting your 'system'.
Post by: Bill S on 16/09/2014 19:29:31
Quote
It depends on how you define "an electron"
It also depends on how you define “place”. In general it must be defined as a position in space. Space must be restricted to the three dimensions that we experience. Any additional dimensions that may be suggested are mathematical concepts which may, or may not, bear any relation to reality.
The question that must arise is: “Can we restrict a quantum object (quon) to the dimensions of the observable – macro – Universe?”
Quantum mechanics works, but no one really knows why. There is something about QM that eludes the best efforts our scientists and mathematicians.
Infinity has similar features. Mathematicians have shown that infinity can be a valuable concept, but there is always something about it that cannot be tamed.
Perhaps looking for a link here is fanciful. Perhaps it should be left to philosophers, but, on the other hand, if it could lead to a clearer understanding of reality; if it could throw some light on some of the apparent paradoxes of the Universe, surely it is worth considering.
Post by: JohnDuffield on 16/09/2014 19:52:03
(https://www.thenakedscientists.com/forum/proxy.php?request=http%3A%2F%2Fupload.wikimedia.org%2Fwikipedia%2Fcommons%2Fthumb%2F4%2F4a%2FDeep_water_wave.gif%2F220px-Deep_water_wave.gif&hash=26300ba8edaa17aa6bb6072600b4767c)
Look under the surface. That wave isn't some localized point-particle thing. It's always in two places at the same time. So is the photon. It's a wave in space, where there is no ocean surface, and it genuinely goes through both slits. But when you detect it at one slit, something like an optical Fourier transform occurs (http://cns-alumni.bu.edu/~slehar/fourier/fourier.html) and the photon is transformed into a dot. This goes through one slit only. Then when you detect it at the screen, something like an optical Fourier transform occurs (http://cns-alumni.bu.edu/~slehar/fourier/fourier.html) and the photon is transformed into a dot. Simple mundane stuff, no magic, and no multiverses.
Post by: David Cooper on 16/09/2014 21:25:15
So, it's complicated stuff, and it's easy for people to disagree while actually believing the same thing and talking at cross purposes. Tighten up your use of language and you might resolve the issue.
Post by: jeffreyH on 16/09/2014 21:27:02
Yor_on, Bill: IMHO if you read up about weak measurement (http://www.physics.utoronto.ca/~aephraim/PWMar13steinberg-final.pdf), you come to appreciate that the Copenhagen Interpretation is old hat, and the photon really is a wave. Have a look at an ocean wave:
(https://www.thenakedscientists.com/forum/proxy.php?request=http%3A%2F%2Fupload.wikimedia.org%2Fwikipedia%2Fcommons%2Fthumb%2F4%2F4a%2FDeep_water_wave.gif%2F220px-Deep_water_wave.gif&hash=26300ba8edaa17aa6bb6072600b4767c)
Look under the surface. That wave isn't some localized point-particle thing. It's always in two places at the same time. So is the photon. It's a wave in space, where there is no ocean surface, and it genuinely goes through both slits. But when you detect it at one slit, something like an optical Fourier transform occurs (http://cns-alumni.bu.edu/~slehar/fourier/fourier.html) and the photon is transformed into a dot. This goes through one slit only. Then when you detect it at the screen, something like an optical Fourier transform occurs (http://cns-alumni.bu.edu/~slehar/fourier/fourier.html) and the photon is transformed into a dot. Simple mundane stuff, no magic, and no multiverses.
I can very much appreciate that point of view.
Post by: Bill S on 16/09/2014 21:44:06
Quote
So, it's complicated stuff, and it's easy for people to disagree while actually believing the same thing and talking at cross purposes.
Absolutely! I think that's very common.
Post by: Bill S on 16/09/2014 21:52:31
Post by: jeffreyH on 16/09/2014 21:59:57
http://en.wikipedia.org/wiki/Meissner_effect
Post by: alancalverd on 16/09/2014 22:50:07
It's nonsense to say that a photon or even an electron is a particle or a wave. It is an entity whose behaviour can be modelled by wave and particle equations. Models are not reality, only a convenient approximation to it.
Post by: jeffreyH on 16/09/2014 23:17:02
Post by: Bill S on 16/09/2014 23:18:18
Post by: jeffreyH on 16/09/2014 23:44:53
Post by: yor_on on 17/09/2014 02:22:02
Found this though: http://www.mpg.de/511738/pressRelease20051011
It's not the exact same as I was thinking of though, but it is interesting.
Post by: JohnDuffield on 17/09/2014 09:30:12
For something to be in one place, the whole of it must be in that place. If some of it is not in that place, that part is in a different place. To say that anything is in two places at the same time is wrong unless the whole of it is in both places at the same time...That's the mistake. Imagine a magnet's magnetic field. It isn't like the green apple. It doesn't have an outside surface. Instead there is no point where it stops. Think of the electron as something like that, minus the magnet in the middle. It's quantum field theory, in QED the electron is an excitation of the electron field. It's "just field", and whilst there might be a middle to it like a hurricane has an eye, it just doesn't make sense to claim that all of it is in one place.
If a green apple is in two places at the same time, it could be sitting on one side of a set of scales while also being in a fruit bowl on a table some distance away. It would be able to balance a red apple on the other side of the scales, an apple of the same mass but which is only located in one place. That doesn't happen though, because when we're dealing with the quantum world......we're dealing with quantum field theory. Everything is fields and waves, and these things just don't have a surface.
So, it's complicated stuff, and it's easy for people to disagree while actually believing the same thing and talking at cross purposes. Tighten up your use of language and you might resolve the issue.I don't think it is complicated actually. I think people make it complicated because they think in terms of billiard balls rather than waves in space.
Post by: evan_au on 17/09/2014 12:49:58
Quote from: jeffreyH
When considering the path of the magnetic field around a superconductor we see a disturbance of the normal behavior. A field is not a particle but we can split it in two.If you can split something in two (and detect that it is split in two) but without changing its nature then you are not dealing with a fundamental object, but with a composite object.
For example, you can split a liter of nitrogen in two without changing its nature, but if I split a nitrogen molecule in two, it has very different properties - so I have reached the fundamental unit of nitrogen gas (at standard temperature & pressure).
There is a fundamental quantised unit of magnetic flux that is found when you cool a superconducting doughnut below its critical temperature in a steady magnetic field. The Meissner effect expels the magnetic field from the superconductor, and the magnetic field through the hole is quantised (http://en.wikipedia.org/wiki/Magnetic_flux_quantum).
If you had two holes, you could detect this quantised flux in one hole or the other - and it will stay "pinned" there until the superconductor heats up again.
Before you measure the flux, there is uncertainty about where the flux resides - but this is classical uncertainty (ie I don't know where it is because I haven't looked yet), not quantum uncertainty (ie I don't know where it is because it is delocalised).
The magnetic fields we normally deal with are far larger than one magnetic flux quantum, giving the illusion that a magnetic field is infinitely divisible (plus, most of us don't deal with superconductors on a daily basis - except maybe alancalvard).
Post by: PmbPhy on 17/09/2014 14:02:31
Quote from: Bill S
Hi Bill,QuoteIt depends on how you define "an electron"
Where did you get this to quote it from? May I request that from now on you let us know whom you're quoting? It makes it easier to let us know who and what you're referring to. Thanks buddy! :)
Post by: PmbPhy on 17/09/2014 14:05:06
Quote from: JohnDuffield
..we're dealing with quantum field theory.The purpose of this thread is quantum mechanics, not quantum field theory. The subject of this thread is about location of particles in space and not fields. If you wish to talk about QFT then please start your own thread. Please don't usurp mine.
Post by: Bill S on 17/09/2014 15:49:14
Sorry, that was a genuine oversight. I was not rashly assuming that those in the thread would necessarily have read it. :) The quote was from Lightarrow: #3.
Post by: JohnDuffield on 17/09/2014 17:23:20
The purpose of this thread is quantum mechanics, not quantum field theory. The subject of this thread is about location of particles in space and not fields. If you wish to talk about QFT then please start your own thread. Please don't usurp mine.It isn't a matter of usurping your thread, it's a matter of getting the physics right. A photon is not a point particle. It has an energy E=hf or E=hc/λ. It has a wavelength. The electron is not a point-particle either. Again, we make electrons (and positrons) out of light waves in pair production (http://en.wikipedia.org/wiki/Pair_production). We can diffract electrons (http://en.wikipedia.org/wiki/Electron_diffraction). In atomic orbitals (http://en.wikipedia.org/wiki/Atomic_orbital) electrons "exist as standing waves". The electron has a magnetic dipole moment (http://en.wikipedia.org/wiki/Electron_magnetic_dipole_moment), and the Einstein-de Haas effect (http://en.wikipedia.org/wiki/Einstein%E2%80%93de_Haas_effect) demonstrates that "spin angular momentum is indeed of the same nature as the angular momentum of rotating bodies as conceived in classical mechanics." And when we annihilate (http://en.wikipedia.org/wiki/Electron%E2%80%93positron_annihilation) the electron with a positron, we typically get two light waves. The evidence is rock-solid, the wave nature of matter is not in doubt, in QED the electron is said to be an excitation of the electron field. It isn't some point particle thing that has a field, its field is what it is. And like the magnet's field, it isn't like an apple. It's always in two places at the same time.
Post by: David Cooper on 17/09/2014 22:09:37
Post by: yor_on on 17/09/2014 22:15:20
"In its totality, nature is therefore dual. None of its constituents can be considered as only a particle or a wave. To reconcile this duality, in 1923 Niels Bohr proposed his Complementarity Principle: simply put, every component in nature has particle-like, as well as wave-like character, and which character is observed at a given time depends only on the observer. In other words, the experiment determines which characteristic one is measuring - particle or wave."
One point here that I find nice is what I read as a wider definition of what a 'observer' should be seen as. "In other words, the experiment determines which characteristic one is measuring - particle or wave.""
There's a big difference between assuming that you need consciousness for the outcome to be set one way or another, and defining it such as the experiment, observer included', all play a part for what the outcome will be. My assumption here though is that retracting any observer, once the experiment is set up, should change nothing in most cases, unless the observer is a integral part of the experiment.
It's a interesting article.
Post by: alancalverd on 18/09/2014 06:58:14
Each fundamental component of the apple then is spread over a wide area, but there are two tightly defined locations where it is more probably going to interact with other things if it is forced to narrow down its range at any time.
As Eddington said, if a physicist fell through the floor and materialised in the room below, he wouldn't consider it a miracle, just a lucky observation of an extremely rare event.
If we make the physicist very small and the floor very thin, we get a tunnel diode - as stocked by most electronics suppliers!
Post by: alancalverd on 18/09/2014 07:10:14
A photon is not a point particle.Oh yes it is. You can capture a single photon on a phtographic plate and it's all there in one place.
Quote
electrons "exist as standing waves".no, their distribution can be modelled as standing waves.
Quote
"spin angular momentum is indeed of the same nature as the angular momentum of rotating bodies as conceived in classical mechanics."No, spin can be adequately modelled as angular momentum but unlike classical angular momentum it is quantised.
Quote
The evidence is rock-solid, the wave nature of matter is not in doubt, in QED the electron is said to be an excitation of the electron field. It isn't some point particle thing that has a field, its field is what it is.No, the behaviour of matter on an atomic scale can be modelled as waves.
Waves and particles are convenient descriptors of things we observe, but the description is not the object and since neither completely describes the behaviour of electrons or photons, neither can be said to be "true" - whatever that means.
I can describe the motion of a car with a Euclidean vector, but it doesn't mean a car is a vector.
Post by: JohnDuffield on 18/09/2014 12:22:22
What is there in an apple that isn't just like an electron or photon?Opposite charge. Simplify the apple to a hydrogen atom. You made this from an electron and a proton. At some distance from either of them their field is readily detectable, and you remember it's QFT and that "the electron is field". But when the electron and the proton come together their opposite fields largely cancel. Now at some distance from the hydrogen atom, the field isn't so readily detectable.
The whole thing can be turned into photons.Yes. And the photon is a wave that isn't localized, like a seismic wave it travels many paths. If you use pair production to convert photons into an electron and positron, you can say that the electron's widespread field isn't localized, and is akin to the many paths. But when you combine the electron with a proton the fields aren't so widespread.
Every part of the the apple is spread across multiple locations (possibly ranging across the entire universe),You can argue that the electron's electromagnetic field is what it is, so the apple's gravitational field is part of what it is. But it has a definable surface, you can pick it up, and throw it, and eat it. People won't accept your argument.
but they're all tied together in such a way that they hold together collectively as an apple, and in an extreme case it could be possible for the probabilities relating to each component of the apple to be in agreement with each other that the whole apple is essentially occupying just two apple-sized locations with high probability while occupying all other possible locations with extremely low probability. Each fundamental component of the apple then is spread over a wide area, but there are two tightly defined locations where it is more probably going to interact with other things if it is forced to narrow down its range at any time.IMHO you should forget about probabilities.
Post by: JohnDuffield on 18/09/2014 12:35:06
Oh yes it is. You can capture a single photon on a phtographic plate and it's all there in one place.Absorbing a wave at some location doesn't mean the wave wasn't an extended entity. Nor does performing an optical Fourier transform on it.
no, their distribution can be modelled as standing waves.Not so. It means they are standing waves. That's why you can diffract them.
No, spin can be adequately modelled as angular momentum but unlike classical angular momentum it is quantised.Again not so. The Einstein-de Haas effect is hard scientific evidence that spin angular momentum is of the same nature as classical angular momentum.
No, the behaviour of matter on an atomic scale can be modelled as waves.You can diffract electrons, you can diffract protons, and neutrons, and buckynalls. Not because they can be modelled as waves, but because of the wave nature of matter.
Waves and particles are convenient descriptors of things we observe, but the description is not the object and since neither completely describes the behaviour of electrons or photons, neither can be said to be "true" - whatever that means.The evidence for the wave nature of matter is overwhelming.
I can describe the motion of a car with a Euclidean vector, but it doesn't mean a car is a vector.But I can make an electron (and a positron) out of electromagnetic waves. Then I can diffract them. Then I can annihilate them, and I've got electromagnetic waves again. That's nothing like describing the motion of a car with a vector.
Post by: lightarrow on 19/09/2014 12:07:08
Ok.It depends on how you define "an electron"It also depends on how you define “place”.
In general it must be defined as a position in space. Space must be restricted to the three dimensions that we experience.
Quote
Any additional dimensions that may be suggested are mathematical concepts which may, or may not, bear any relation to reality.If you think that only a three-dimensional world is "real" because "we experience this one only", and that the 4-dimensional world of GR (for example) is "just an idea", then you are wrong: it simply depends on how you interpret your perceptions; even "three-dimensional" is a concept just in our minds, "the reality" doesn't exist.
Quote
The question that must arise is: “Can we restrict a quantum object (quon) to the dimensions of the observable – macro – Universe?”And instead why classical mechanics works? I can simplify my question to the question: why Hamilton's principle of mechanics works? (As you know, all most important things of mechanics come from that principle).
Quantum mechanics works, but no one really knows why.
In QM there are more than one principle; so who asks why QM works should ask "why do those principles works?" Or the problem is the fact that here there are more than one principle? Then you should better ask: "Is it possible to find an unique principle for QM as there is in classical mechanics?"
It was this the real question?
--
lightarrow
Post by: David Cooper on 19/09/2014 17:02:03
IMHO you should forget about probabilities.
Why would I want to forget about probabilities? The probabilities are determined by the distribution of the item that is spread out across a space, dictating how likely it is that that item will appear as a point particle at any position within that space if it is forced to choose a location to do so. Until it is forced to choose, it is able to maintain multiple possibilities as to where that point will be based on its distribution. Things are spread out while they keep the possibilities open, but there are times when they are forced to narrow down that range (though not necessarily to a single possibility). The result is that when a photon or electron is forced to interact with something in a way that requires it to have a tightly constrained location, it will then appear to exist at that single location. We can work out where it is most likely to do so based on probabilities, but is fully entitled to take us by surprise by selecting the most improbable option and appear at the other side of the universe instead. The apple can do the same thing, but the odds are stacked rather more heavily against it appearing anywhere other than where we most expect it to be, to the point that we can confidently state where it is with a practical guarantee (though not an absolute one) of being right.
Now, you seem to have decided that when an electron and proton get together (let's go the whole hog and combine them to make a neutron), the fields cancel out and result in something that is no longer spread out in the way that an electron is, but can that really be so? An experiment has been done in which an object made up of many atoms was oscillating in two opposite directions at the same time, and by that I mean that it was moving from right to left to right to left while also moving from left to right to left to right at the same time. I forget the details as to how the scientists proved that it was doing this, but it was held up as a demonstration of quantum effects with objects on the macro scale. That means we have large physical compound objects (not unlike an apple) behaving as if they are spread out and wavy. If you interact with the thing, you then force it to reduce the range of what it is doing and to pick only one of the two options, but up to that point it was doing both, and at many points along the way the end of this oscillating thing was "in two different places at the same time", in much the same way as Schrödinger's cat is both alive and dead at the same time (one walking around while the other is lying still), and in much the same way as my apple was in two places at the same time.
Everything I've just said may be hogwash, of course, because I'm not an expert in this at all. I merely listen to what more knowledgeable people say, probably misunderstand a great deal of it, try to build a model of what I think they mean in my head, and then I come to places like this to see if people who know more than I do can spot the points where I've gone wrong, and then I hopefully improve my understanding as a result. So, I'm always delighted to be shown to be wrong.
Post by: alancalverd on 19/09/2014 17:06:46
But I can make an electron (and a positron) out of electromagnetic waves. Then I can diffract them. Then I can annihilate them, and I've got electromagnetic waves again. That's nothing like describing the motion of a car with a vector.
So you are now asserting that electromagnetic waves have mass?
Post by: jeffreyH on 19/09/2014 18:29:49
Post by: JohnDuffield on 19/09/2014 18:31:14
So you are now asserting that electromagnetic waves have mass?No. I'm referring to pair production.
(http://en.wikipedia.org/wiki/Pair_production)
Quote from: David Cooper
Why would I want to forget about probabilities?Because weak measurement work by Aephraim Steinberg et al (http://www.physics.utoronto.ca/~aephraim/) and by Jeff Lundeen et al (http://www.photonicquantum.info/) demonstrate that wavefunction is real and that the Copenhagen interpretation is passé. See this (http://www.photonicquantum.info/Research/SemiTechnical_Wavefunction.html):
"With weak measurements, it’s possible to learn something about the wavefunction without completely destroying it. As the measurement becomes very weak, you learn very little about the wavefunction, but leave it largely unchanged. This is the technique that we’ve used in our experiment. We have developed a methodology for measuring the wavefunction directly, by repeating many weak measurements on a group of systems that have been prepared with identical wavefunctions. By repeating the measurements, the knowledge of the wavefunction accumulates to the point where high precision can be restored.
So what does this mean? We hope that the scientific community can now improve upon the Copenhagen Interpretation, and redefine the wavefunction so that it is no longer just a mathematical tool, but rather something that can be directly measured in the laboratory."
Quote from: David Cooper
The probabilities are determined by the distribution of the item that is spread out across a space, dictating how likely it is that that item will appear as a point particle at any position within that space if it is forced to choose a location to do so. Until it is forced to choose, it is able to maintain multiple possibilities as to where that point will be based on its distribution. Things are spread out while they keep the possibilities open, but there are times when they are forced to narrow down that range (though not necessarily to a single possibility).The item is wavefunction, it's distributed in space, and it's real, not some abstract probabilistic thing.
Quote from: David Cooper
The result is that when a photon or electron is forced to interact with something in a way that requires it to have a tightly constrained location, it will then appear to exist at that single location.No problem with that. When you detect the photon at one of the slits it's as if you've done an optical Fourier transform (http://cns-alumni.bu.edu/~slehar/fourier/fourier.html) so it goes through that slit only. When you detect it at the screen again it's as if you've performed an optical Fourier transform, and you see a dot on the screen. But the photon always had its E=hf = hc/λ wave nature.
Quote from: David Cooper
We can work out where it is most likely to do so based on probabilities...And underlying those probabilities is something real. The Copenhagen interpretation basically says "you can never hope to understand it". Well, we can.
Quote from: David Cooper
Now, you seem to have decided that when an electron and proton get together (let's go the whole hog and combine them to make a neutron), the fields cancel out and result in something that is no longer spread out in the way that an electron is, but can that really be so?Yes. That's why the hydrogen atom mass is less than that of the free electron plus that of the free proton. The fields don't quite cancel, but because they largely cancel, the hydrogen is more localised than either the electron or the proton.
Quote from: David Cooper
An experiment has been done in which an object made up of many atoms was oscillating in two opposite directions at the same time, and by that I mean that it was moving from right to left to right to left while also moving from left to right to left to right at the same time.That's what a photon in a cavity does. It's a wave thing.
Quote from: David Cooper
I forget the details as to how the scientists proved that it was doing this, but it was held up as a demonstration of quantum effects with objects on the macro scale. That means we have large physical compound objects (not unlike an apple) behaving as if they are spread out and wavy. If you interact with the thing, you then force it to reduce the range of what it is doing and to pick only one of the two options, but up to that point it was doing both, and at many points along the way the end of this oscillating thing was "in two different places at the same time", in much the same way as Schrödinger's cat is both alive and dead at the same time (one walking around while the other is lying still), and in much the same way as my apple was in two places at the same time.Schrödinger devised his cat to demonstrate the stupidity of the Copenhagen interpretation. But it got hijacked by people who promote quantum mysticism.
Quote from: David Cooper
Everything I've just said may be hogwash, of course, because I'm not an expert in this at all. I merely listen to what more knowledgeable people say, probably misunderstand a great deal of it, try to build a model of what I think they mean in my head, and then I come to places like this to see if people who know more than I do can spot the points where I've gone wrong, and then I hopefully improve my understanding as a result. So, I'm always delighted to be shown to be wrong.Some of what you're saying is somewhat outdated. The thing is that some "expert" who's been banging on about the Copenhagen Interpretation for forty years isn't going to tell you about the weak measurement work. It was awarded the top two slots in the Institute of Physics "physicsworld" breakthroughs of 2011 (http://physicsworld.com/cws/article/news/2011/dec/16/physics-world-reveals-its-top-10-breakthroughs-for-2011). But even now hardly anybody has heard about it.
Post by: jeffreyH on 19/09/2014 18:43:05
"Schrödinger devised his cat to demonstrate the stupidity of the Copenhagen interpretation. But it got hijacked by people who promote quantum mysticism."
It's about time people realized this!!
Post by: yor_on on 19/09/2014 20:13:58
=
Myself I consider it a try for a Newtonian world image, in where we have guaranteed forces that act upon us, a clock work universe. Which makes it doubly ironic to presume that action and reaction isn't there in the measurement.
Post by: yor_on on 19/09/2014 20:40:18
Post by: PmbPhy on 19/09/2014 21:01:14
From John Duffield.That's always been the case, Jeff. E.g. on page 431 of Introduction to Quantum Mechanics - Second Edition by David Griffiths, (2005) the author writes
"Schrödinger devised his cat to demonstrate the stupidity of the Copenhagen interpretation. But it got hijacked by people who promote quantum mysticism."
It's about time people realized this!!
Quote
Schrodinger regarded this as patent nonsense, and I think that most physicists would agree with him. There is something absurd about the very idea of a macroscopic object being in a linear combination of two palpably different states. An electron can be in be in a linear combination of spin up and spin down, but a cat simply cannot be in a linear combination of alive and dead.I recommend that you read the entire section of this book on the topic rather than a mere "sound bite". It's in section 12.4, pages 430 to 431.
Post by: PmbPhy on 19/09/2014 21:05:17
If the electron can be in two places at once then the two places can be anywhere including right next to each other. If they are right next to each other the Pauli Exclusion principle makes them into entirely different electrons. They are then not identical. If every electron in the universe can be considered to be in two places at once then all pairings become unique. So what is to stop these two unique objects from also being in two places at once. On and on ad infinitum.Not so. Two electrons can't be in the same quantum state. That means that no two electrons can have overlapping wave functions and the same spin and be in the same state. Besides, that only applies to electrons. I merely used electrons as an example for the thread. People claim that this whole idea applies to any two identical particles whatsoever.
Post by: PmbPhy on 19/09/2014 21:18:02
Quote from: alancalverd
So you are now asserting that electromagnetic waves have mass?Although I rarely, if ever, agree with John and I don't think that's what he's saying, there's nothing intrinsically wrong with the notion that EM waves have mass. Einstein himself proved this a long time ago. Are you not familiar with his paper on the subject? If not then please see
http://home.comcast.net/~peter.m.brown/sr/einsteins_box.htm
It's based on the article The Principle of Conservation of the Center of Gravity and the Inertia of Energy, Albert Einstein, Annalen der Physik, 20 (1906): 626-633.
Quote
…, if one assumes that any energy E possesses the inertia E/c2, then the contradiction with the principle of mechanics disappears. For according to the this assumption the carrier body of mass E/c2, while it transports the amount of energy E from B to A; and since the center of gravity of the entire system must be at rest during this process according to the center-of-mass theorem the cylinder K undergoes during it a total shift S’ to the right.. If we assign the electromagnetic field too a mass density
Consider also what Einstein said in is 1916 paper on GR tooQuoteWe make the distinction hereafter between "gravitational field" and "matter" in this way, that we denote everything but the gravitational field as "matter." Our use of the word therefore includes not only matter in the ordinary sense, but the electromagnetic field as well.
...
The special theory of relativity has led to the conclusion that inert mass is nothing more or less than energy, which finds its complete mathematical expression in a symmetrical tensor of second rank, the energy tensor.
Post by: yor_on on 19/09/2014 21:40:42
“Prof. Dmitri Petrov in collaboration with colleagues from the Moscow State University were the first to measure this effect. To measure the effect the researchers use a photonic force microscope (PFM) to trap a small metal covered dielectric sphere in a laser beam. The surrounding liquid contains fluorescent molecules that attach to the surface of the sphere. Excited by the light of the laser the molecules themselves start to emit light. Just like a soldier feels the recoil of his gun after firing a projectile, the small molecules pass their momentum they get from light emission on to the sphere. During this process the scientists measure two values: the forces acting on the bead (described by a tiny dithering in the trap) and the intensity of light generated by the molecules coating the bead's surface. As the intensity of the emitted light fades over time due to bleaching the scientists can observe a decline of the recoil as well.
What initially was the set-up for a different experiment turned out to deliver the first direct proof of the correlation between light emission and recoil and also allowed the calculation of the power of light emitted. In their experiment the researches measured a force of 240 femtonewtons, which equals a power coming from the bead of 1 microwatt. "Until now it has been really difficult to say how much light eventually comes off this material", says Dmitri Petrov from ICFO, “but by looking at the recoil we have a completely new approach to quantify light emission by a mechanical force”. Possible applications of the PFM-setup could be to offer a more precise way of measuring the efficiency and intensity of other light-emitting molecules, including the bleaching of fluorescent dyes.”
Let us put it this way John, assume that the 'force carriers' in your weak experiments are photons, there has to be something that communicate a 'nudge' or whatever it is one want to measure or influence. Above is an example of action and reaction, created in the interaction of matter and photons.
you can explain it different ways, it could be seen as a necessary symmetry, which I like, instead of a proof of a 'path'. But, no matter how I like to define it, it still is a example of 'action and reaction' to me, and the recoil defined to a photon. So what communicates in those weak experiments?
Post by: David Cooper on 19/09/2014 22:03:20
The item is wavefunction, it's distributed in space, and it's real, not some abstract probabilistic thing.
I never said it wasn't real. The probabilities are real too, dictated by a real mechanism which I have never denied (because I have never believed in magic and have never been a fan of the Copenhagen interpretation). Recent advances are enabling the mechanism to be detected, as anyone on this forum can't fail to have noticed, but it makes no change whatsoever to the issue of whether something is in two places or not at the same time. A thing can be spread across multiple places, but it cannot in totality be in two places at the same time.
So, I'm still trying to determine whether there's a real issue in conflict between what you're saying and what I've been led to believe. Brian Cox recently discussed a diamond in a locked box and the possibility of it suddenly appearing outside the box because the probability of this unlikely event occurring is supposedly not quite being zero. This would, I assume, depend on the diamond behaving in a wavy way such that every part of it too is spread out across space and all of those parts could spontaneously decide that they are outside the box instead of in it. So, do you disagree with him and think the diamond is so localised that it cannot get out of the box in such a way at all? The whole diamond is wavefunction and it enables the diamond to show itself as being located outside the box even though it was seen as being located inside it when it was locked in there: mechanism and probabilities intact.
Post by: PmbPhy on 19/09/2014 22:54:54
Quote from: David Cooper
Brian Cox recently discussed a diamond in a locked box and the possibility of it suddenly appearing outside the box because the probability of this unlikely event occurring is supposedly not quite being zero.I disagree with this kind of response to the problem of macroscopic problems and probabilities. I believe that it's quite literally impossible for that to happen, not just a small probability.
I checked this with the author of the textbook which I learned QM from saying that I don't buy that kind of thing for a moment and asked him if he did. His response was I guess so, but it's a silly remark, designed to mislead.
Keep that in mind when people make those kinds of assertions.
Post by: JohnDuffield on 20/09/2014 15:00:39
I never said it wasn't real. The probabilities are real too, dictated by a real mechanism which I have never denied (because I have never believed in magic and have never been a fan of the Copenhagen interpretation).Good stuff.
Recent advances are enabling the mechanism to be detected, as anyone on this forum can't fail to have noticed, but it makes no change whatsoever to the issue of whether something is in two places or not at the same time. A thing can be spread across multiple places, but it cannot in totality be in two places at the same time.Fair enough. IMHO the important point is that something like a photon is more like a seismic wave than a billiard ball.
So, I'm still trying to determine whether there's a real issue in conflict between what you're saying and what I've been led to believe. Brian Cox recently discussed a diamond in a locked box and the possibility of it suddenly appearing outside the box because the probability of this unlikely event occurring is supposedly not quite being zero. This would, I assume, depend on the diamond behaving in a wavy way such that every part of it too is spread out across space and all of those parts could spontaneously decide that they are outside the box instead of in it. So, do you disagree with him and think the diamond is so localised that it cannot get out of the box in such a way at all? The whole diamond is wavefunction and it enables the diamond to show itself as being located outside the box even though it was seen as being located inside it when it was locked in there: mechanism and probabilities intact.Yes, I disagree with him. See pmb's response above. Brian Cox has done some nice TV programs, but IMHO he doesn't always get the physics right.
Post by: evan_au on 20/09/2014 22:27:53
Post by: alancalverd on 21/09/2014 00:09:53
Fair enough. IMHO the important point is that something like a photon is more like a seismic wave than a billiard ball.
You can propagate a billiard ball, but not a seismic wave, through a vacuum
Post by: Bill S on 08/12/2014 20:48:29
http://www.askamathematician.com/2009/12/q-can-things-really-be-in-two-places-at-the-same-time
Post by: PmbPhy on 08/12/2014 22:10:21
Pete, although this is not the source of the idea it is certainly one of the dissemination points.I myself reject what's said in that page. The wave function tells the probability of where a particle will end up. To say that a photon interferes with itself is to say that the wave "is" the photon instead of merely being a tool to tell you probability.
http://www.askamathematician.com/2009/12/q-can-things-really-be-in-two-places-at-the-same-time
Post by: JohnDuffield on 08/12/2014 23:20:51
As far as we know you can propagate a gravitational wave through a vacuum. Have a read of the LIGO website (http://www.ligo-la.caltech.edu/LLO/overviewsci.htm):Fair enough. IMHO the important point is that something like a photon is more like a seismic wave than a billiard ball.You can propagate a billiard ball, but not a seismic wave, through a vacuum
"When large masses move suddenly, some of this space-time curvature ripples outward, spreading in much the way ripples do the surface of an agitated pond. Imagine two neutron stars orbiting each other. A neutron star is the burned-out core often left behind after a star explodes. It is an incredibly dense object that can carry as much mass as a star like our sun, in a sphere only a few miles wide. When two such dense objects orbit each other, space-time is stirred by their motion, and gravitational energy ripples throughout the universe..."
OK an electromagnetic wave isn't a gravitational wave, but you must have read Maxwell saying light consists of transverse undulations. Light is a wave, photon energy is E=hc/λ, it's λ for wavelength. It's a wave, and it's moving through space. When an ocean wave moves through the sea, the sea waves. When a seismic wave moves through the ground, the ground waves. When an electromagnetic wave moves through space...
Note that you can diffract electrons. Electrons are waves too. The dual slit experiment can be easily explained if detection at a slit or the screen performed something akin to an optical Fourier transform and converted an extended-entity wave into a dot.
Bill: I reject the Copenhagen Interpretation along with the idea that the wavefunction is some probabilistic mystic thing. See Jeff Lundeen's website here (http://www.photonicquantum.info/) and catching sight of the elusive wavefunction (http://physicsworld.com/cws/article/news/2011/jun/15/catching-sight-of-the-elusive-wavefunction) and this article (http://www.photonicquantum.info/Research/SemiTechnical_Wavefunction.html) where he says wavefunction is real.
Post by: Bill S on 09/12/2014 15:33:17
Quote from: Pete
To say that a photon interferes with itself is to say that the wave "is" the photon instead of merely being a tool to tell you probability.
Now I’m confused. I know that’s not unusual, but perhaps we can sort it this time. [:P]
I doubt that you are saying that a photon does not have characteristics of a wave. So when you say the wave is “a tool to tell you probability”, are you referring to the wave function, as distinct from the wavelike characteristic of a photon?
Post by: PmbPhy on 09/12/2014 15:43:37
The wave function describes exactly what the wavelike characteristic of a photon is, i.e. the probability of where to find the photon.Quote from: PeteTo say that a photon interferes with itself is to say that the wave "is" the photon instead of merely being a tool to tell you probability.
Now I’m confused. I know that’s not unusual, but perhaps we can sort it this time. [:P]
I doubt that you are saying that a photon does not have characteristics of a wave. So when you say the wave is “a tool to tell you probability”, are you referring to the wave function, as distinct from the wavelike characteristic of a photon?
Post by: lightarrow on 09/12/2014 19:17:23
The photon is a particle described by quantum electrodynamics and as such it's described using a "wavefunction" exactly as the electron is (not the same wavefunction, but still a wavefunction). Maybe you are confusing a photon with the electromagnetic field, which instead is described by a real wave (not a "wavefunction")?Quote from: PeteTo say that a photon interferes with itself is to say that the wave "is" the photon instead of merely being a tool to tell you probability.
Now I’m confused. I know that’s not unusual, but perhaps we can sort it this time. [:P]
I doubt that you are saying that a photon does not have characteristics of a wave. So when you say the wave is “a tool to tell you probability”, are you referring to the wave function, as distinct from the wavelike characteristic of a photon?
--
lightarrow
Post by: PmbPhy on 09/12/2014 23:27:20
Quote from: lightarrow
The photon is a particle described by quantum electrodynamics and as such it's described using a "wavefunction" exactly as the electron is (not the same wavefunction, but still a wavefunction). Maybe you are confusing a photon with the electromagnetic field, which instead is described by a real wave (not a "wavefunction")?I'm happy you said that, lightarrow. If you hadn't then I'd accept what Wikipedia had to say about it, i.e.
http://en.wikipedia.org/wiki/Photon
Quote
Like all elementary particles, photons are currently best explained by quantum mechanics and exhibit wave–particle duality, exhibiting properties of both waves and particles.And I'd have accepted it. However you challenge me quite often which keeps me sharp so I contacted a friend of mine (an author of a QM text) and he said
Quote
I don't think so. Photons really have no place in (nonrelativistic) quantum mechanics, since they are quintessentially relativistic entities.So although he's not 100% certain he makes a good point in that photons are relativistic particles and thus they belong to a relativistic theory which quantum mechanics is not since it's a non-relativistic theory.
So please keep up the good work my friend. :)
Post by: yor_on on 13/12/2014 20:23:23
Would expect nothing less of you Lightarrow :)
Imagination is all, then comes proof.
Post by: Bill S on 17/12/2014 20:02:28
Quote from: Bill S.
It also depends on how you define “place”.
In general it must be defined as a position in space. Space must be restricted to the three dimensions that we experience.
and:
Quote
Any additional dimensions that may be suggested are mathematical concepts which may, or may not, bear any relation to reality.
So Lithtarrow’s comment:
Quote from: Lightarrow
If you think that only a three-dimensional world is "real" because "we experience this one only", and that the 4-dimensional world of GR (for example) is "just an idea", then you are wrong: it simply depends on how you interpret your perceptions; even "three-dimensional" is a concept just in our minds, "the reality" doesn't exist.
while, in itself, accurate, is not appropriate.
The 4-dimensional world of GR refers to spacetime, not just to space. What I meant was that any additional dimensions of space were just mental concepts.
I acknowledge that our three spacial dimensions may also be mental concepts, but they are concepts of which we have a physical perception; which would not be the case for a 4th spacial dimension.
Post by: Bill S on 17/12/2014 20:14:53
“Can an electron be in two places at the same time?”
As learning is my primary objective, I would really appreciate your input on this.
Post by: Bill S on 10/02/2015 22:06:17
This seems to have some interesting stuff that is relevant to the OP. It is almost 10 years old, so I suppose it could be out of date.
Post by: jeffreyH on 11/02/2015 00:04:41
Post by: jccc on 11/02/2015 01:54:24
All is connected, all is confused.
I need my pipe.
I should be in your place by now.
Post by: Ethos_ on 11/02/2015 02:14:44
There is one place, called space. There is one time, called now.Actually Jccc, one can't separate space and time when defining a location. Physicists refer to this 4 dimensional reality as space/time. And the reason why it takes more than 3 dimensions to describe reality is that one moment ago, you were in a different location than you are at present. Not only are there 3 spatial dimensions of linear measure which describe a location, but that location changes as time advances to another location in the future. And to make another important point, absolute motionlessness is only possible when one frame of reference is considered for a pair of objects. One of the objects can be considered motionless relative to the other. But nothing is motionless relative to space, everything is moving thru space/time.
All is connected, all is confused.
I need my pipe.
I should be in your place by now.
Post by: PmbPhy on 11/02/2015 02:27:53
Post by: jccc on 11/02/2015 02:34:39
Let's remember that the assertion that a particle can be in two places at the same time is quite wrong.
How about a thing can be particle and wave at the same time?
Post by: Ethos_ on 11/02/2015 02:38:30
Let's remember that the assertion that a particle can be in two places at the same time is quite wrong.Absolutely Pete,..............and that's because it is never the same time for any two separate locations. Space/time is a composite and one has no real meaning without the other.
One cannot separate space and time when defining a location,"place".
Post by: PmbPhy on 11/02/2015 02:45:17
Quote from: jccc
How about a thing can be particle and wave at the same time?That's not something found in quantum mechanics. In fact it says that, for example, an electron, photon etc. can have particle properties and it can have wave properties, depending on what experiment is being performed. But they cannot have both properties at the same time.
See http://abyss.uoregon.edu/~js/21st_century_science/lectures/lec15.html
Quote
Wave-particle duality does not mean that a photon or subatomic particle is both a wave and particle simultaneously, but that it could manifest either a wave or a particle aspect depending on circumstances.
...
No experiment can ever measure both aspects simultaneously and so we never see a mixture of wave and particle.
Post by: JohnDuffield on 11/02/2015 13:53:38
Actually Jccc, one can't separate space and time when defining a location. Physicists refer to this 4 dimensional reality as space/time. And the reason why it takes more than 3 dimensions to describe reality is that one moment ago, you were in a different location than you are at present. Not only are there 3 spatial dimensions of linear measure which describe a location, but that location changes as time advances to another location in the future. And to make another important point, absolute motionlessness is only possible when one frame of reference is considered for a pair of objects. One of the objects can be considered motionless relative to the other. But nothing is motionless relative to space, everything is moving thru space/time.I'm somebody who roots for relativity, but I don't share this view at all. Take a look at A World without Time: The Forgotten Legacy of Godel and Einstein (https://www.google.co.uk/?gws_rd=ssl#q=%22no+motion+in+spacetime%22+). The reality is space and motion. That's motion through space, not spaceitme. Spacetime is a combination of space and time, but it's an abstract thing. The map is not the territory. You cannot move through spacetime because it represents space at all times. When some particle is motionless in space, as determined from the CMB rest frame (http://en.wikipedia.org/wiki/Cosmic_microwave_background#CMBR_dipole_anisotropy), it isn't actually moving through spacetime. Other particles are moving through space, that's all.
Let's remember that the assertion that a particle can be in two places at the same time is quite wrong.I must take exception to this. Its quantum field theory, not quantum point particle theory. The electron's field is what it is, and it doesn't have an edge. Saying a particle can't be in two places at once makes as much sense as saying the wind can't be in two places at once.
Post by: Ethos_ on 11/02/2015 15:00:29
The reality is space and motion. That's motion through space, not spaceitme. Spacetime is a combination of space and time, but it's an abstract thing.Are you suggesting that we should not view time as the fourth dimension? Because if your idea is correct, space/time would only be an abstraction.
The dimensions of space are evident and we need no motion to understand their reality. And they consist of three dimensions.
Motion is the evidence that the fourth dimension is also a reality. And the path thru it is as real as the other three.
I must respectfully disagree with your position on this issue John.
Post by: jccc on 11/02/2015 15:52:31
The reality is space and motion. That's motion through space, not spaceitme. Spacetime is a combination of space and time, but it's an abstract thing.Are you suggesting that we should not view time as the fourth dimension? Because if your idea is correct, space/time would only be an abstraction.
The dimensions of space are evident and we need no motion to understand their reality. And they consist of three dimensions.
Motion is the evidence that the fourth dimension is also a reality. And the path thru it is as real as the other three.
I must respectfully disagree with your position on this issue John.
Still think light is particle?
Post by: Bill S on 11/02/2015 17:21:45
Post by: Ethos_ on 11/02/2015 17:42:08
John. you were not directing us to Louis Savain, were you? [:D]Louis Savain...........Rebel Scientist?......or Crackpot!
Post by: PmbPhy on 11/02/2015 21:03:33
Quote from: jccc
Still think light is particle?You've never been in a laboratory and watched or ran these experiments so you don't know what you're talking about. Anybody who knows the results knows that if the light source is dim enough then light arrives at the screen in clumps. That means that it appears as specks of light, e.g. the light is localized in space and interacts with light detectors in a finite localized location, not spread out all over the place. The stronger the source of light the more it appears to be spread out in space like a wave.
Post by: Bill S on 12/02/2015 16:02:16
“But if both slits are open, the wave function for the electron penetrating screen S1 is the superposition of states, (ψ1 + ψ2), so that the probability density for the electron wave reaching screen S2 is |(ψ1 + ψ2)|2 = |ψ1|2 + |ψ2|2 + (ψ 1*ψ2 + ψ1ψ 2*). The first two terms above are the partial probability densities that the electron will pass through slit s1 or slit s2. The third term (the cross product) is the ‘interference’ part of the scattering. It shows up on screen S2 as a diffraction pattern.”
I understand that this explains the diffraction pattern produced by electrons, and I know (in a general way) what ψ1 and ψ2 are. However I run into trouble with the next bit of math [|(ψ1 + ψ2)|2 = |ψ1|2 + |ψ2|2 + (ψ 1*ψ2 + ψ1ψ 2*).] especially the last part.
I would really appreciate a guide for the mathematically challenged.
Post by: JohnDuffield on 12/02/2015 18:12:16
Are you suggesting that we should not view time as the fourth dimension? Because if your idea is correct, space/time would only be an abstraction.I'm suggesting you should view time as a dimension in the sense of measure, but not in the sense of freedom of motion. Time isn't on a par with space. In a metric signature we say (-+++). I can hop forward a metre but you can't hop forward a second.
The dimensions of space are evident and we need no motion to understand their reality. And they consist of three dimensions.No problem.
Motion is the evidence that the fourth dimension is also a reality.There's no problem with motion. That's evident too. But note that a clock "clocks up" some kind of regular cyclical motion and shows you some cumulative result called the time. It's a cumulative measure of motion. When some guy in some science fiction movie has some gizmo to stop time, what he actually stops is motion.
And the path thru it is as real as the other three.But you can't move through motion. You can move through space, but not through motion, and not through time, and not through spacetime. Louis Savain is right about that.
Post by: yor_on on 12/02/2015 18:57:43
The reality is space and motion. That's motion through space, not spaceitme. Spacetime is a combination of space and time, but it's an abstract thing.Are you suggesting that we should not view time as the fourth dimension? Because if your idea is correct, space/time would only be an abstraction.
The dimensions of space are evident and we need no motion to understand their reality. And they consist of three dimensions.
Motion is the evidence that the fourth dimension is also a reality. And the path thru it is as real as the other three.
I must respectfully disagree with your position on this issue John.
Still think light is particle?
It's a duality, depending on observer and experiment. If you want everything involved to become 'observers' of each other, then that's ok by me.
Post by: Ethos_ on 12/02/2015 18:59:55
But you can't move through motion. You can move through space, but not through motion, and not through time, and not through spacetime.I wasn't suggesting that one could move through "motion", that would make no sense. But it is possible to move through time. We are moving through time as I write this sentence. And we can also move through it in other more dramatic ways. Time dilation has been recognized for many years and proved with the advent of space travel. When you make the remark that we can't move through time, I find myself wondering how you could make such a statement. You are obviously an intelligent individual judging from your previous posts on different subjects. But I am frankly astounded to hear you make the statement; "You can move through space but not through time."
Post by: jeffreyH on 12/02/2015 19:21:28
Post by: JohnDuffield on 12/02/2015 19:31:56
I wasn't suggesting that one could move through "motion", that would make no sense. But it is possible to move through time. We are moving through time as I write this sentence.Cross my heart and hope to die Ethos, that's just a figure of speech. Let's forget about the motion of the Earth and the Sun and the Galaxy. Let's say you're sitting in your chair, motionless. Why do you think you're "moving through time"? Because all around you, things are moving. If I were to snap my gedanken fingers and stop all the motion inside your body and brain such that you were in total stasis (http://en.wikipedia.org/wiki/Stasis_(fiction)), then when I snapped my fingers again a hundred years later, you might claim you'd travelled through time. But you didn't actually travel through time. You didn't travel anywhere. You didn't move at all. Instead everything else did. And that motion was through space.
And we can also move through it in other more dramatic ways. Time dilation has been recognized for many years and proved with the advent of space travel.There's no issue with time dilation. That's where your rate of local motion is less than mine. But it isn't time travel. You don't end up in the middle of last week. You could set off in some fast rocket on some out and back trip, and I could watch you every step of the way. You never leave the present.
When you make the remark that we can't move through time, I find myself wondering how you could make such a statement. You are obviously an intelligent individual judging from your previous posts on different subjects. But I am frankly astounded to hear you make the statement; "You can move through space but not through time."It's true Ethos. I can hold my hands up a foot apart and show you the gap, the space between them. I can also waggle my hands and show you motion - my hand is at one location, then another, et cetera, and you can be confident that my hand is moving through space. But if I stop moving my hand, in what sense is it moving through time? It's just a figure of speech I'm afraid. That's what A World without Time: The Forgotten Legacy of Godel and Einstein (http://www.amazon.co.uk/World-without-Time-Forgotten-Einstein/dp/0465092942) is all about. Time is something like heat. Heat is real. So is time. A hundred years will kill you just as surely as a hundred degrees C. But just as you don't literally climb to a higher temperature, you don't literally travel through time.
Post by: JohnDuffield on 12/02/2015 19:35:44
John. you were not directing us to Louis Savain, were you? [:D]No, I meant to be directing you to A World without Time: The Forgotten Legacy of Godel and Einstein (http://www.amazon.co.uk/World-without-Time-Forgotten-Einstein/dp/0465092942). But my URL was a google search on "no motion in spacetime". My bad. Louis Savain has Einstein on his crackpot list, saying this:
"I placed Albert Einstein at the bottom of the list because he, of all people, should have known better. The man needs no introduction, of course, but why is he on the list? Because he (reluctantly but who cares?) agreed with his good friend, Kurt Gödel, that the spacetime of general relativity allows time travel to the past via closed time-like loops..."
That's wrong. In A World without Time you can read how Gödel worked out that time cannot pass if you can visit the past, and that time travel is not possible. Wheeler conflated a temporal circle with a cycle. You can't actually travel around a closed-timelike curve, just as you can't actually fly along a worldline.
Jeffrey: your post noted.
Post by: Bill S on 12/02/2015 19:51:56
Quote from: John
You can't actually travel around a closed-timelike curve,
Amen!!!! Instinctively I believed that, but knowing that neither belief, nor instinct, leads to good science (unless you are Einstein) I have devoted a lot of time and effort to trying to prove myself wrong. No success yet.
Post by: chiralSPO on 12/02/2015 20:00:49
I think it is a very important point, though, to consider that typically there is little relative "motion" along the timeline, and we only observe "now" so it is usually pretty useless to think about the time coordinates of objects. It is useful to think about the time coordinates of events though...
Post by: Ethos_ on 12/02/2015 20:03:56
But just as you don't literally climb to a higher temperature, you don't literally travel through time.OK,......but would it then be accurate to say: "The advance of time has moved the location of our reality." Because for time to be a dimension, it should progress in a linear fashion.
Post by: Bill S on 12/02/2015 20:18:30
Quote from: Ethos
Because for time to be a dimension, it should progress in a linear fashion.
I'm not sure I follow the logic of that. Tensed time should progress, but tensless (untensed) time has more in common with spatial dimensions, and that is static.
Post by: Ethos_ on 12/02/2015 20:20:52
Motion through time is a potentially unfortunate figure of speech. But in some sense it is correct. If we think of spacetime as a four-dimensional (mathematical) space, where any point is defined by three spatial coordinates and one temporal coordinate, and if we consider changing an object's spatial coordinates as motion through space, then why not consider changing the temporal coordinate as motion through time?Well said my friend. The passage of these events is how we understand the change and as those changes take place, we experience them passing before us. It may be a little off mark to suggest motion through time but time is surely passing. And as it passes, it is moving us to a new reality.
I think it is a very important point, though, to consider that typically there is little relative "motion" along the timeline, and we only observe "now" so it is usually pretty useless to think about the time coordinates of objects. It is useful to think about the time coordinates of events though...
Post by: Ethos_ on 12/02/2015 20:31:35
The reason I find that logical comes from; The passage of time is a collection of changing events in a region lying between the past and the future. Just as the region lying between two points of width, length, or height are dimensions, so is the region lying between the past and future. And as such, a linear dimension as defined by a region lying between two points or locations. Time qualifies in that respect because each moment, each Planck moment places us in a different location in time. And because space and time can't be logically separated, a new location in space/time.Quote from: EthosBecause for time to be a dimension, it should progress in a linear fashion.
I'm not sure I follow the logic of that.
Post by: Bill S on 14/02/2015 18:43:59
Post by: JohnDuffield on 15/02/2015 14:37:02
Amen!!!! Instinctively I believed that, but knowing that neither belief, nor instinct, leads to good science (unless you are Einstein) I have devoted a lot of time and effort to trying to prove myself wrong. No success yet.I think Groundhog Day is illuminating. If I were to propose some closed timelike curve where the worldline is 24 hours long, most people think they'd live the same day over and over again. But that isn't how it is. It's more like your life lasted 24 hours. So it's more like Mayfly Day. Only it's causeless. Like you hatched from your own egg or something equally weird. In no way does this rather surreal abstract notion offer any prospect of time travel.
Post by: JohnDuffield on 15/02/2015 15:09:32
Motion through time is a potentially unfortunate figure of speech. But in some sense it is correct. If we think of spacetime as a four-dimensional (mathematical) space, where any point is defined by three spatial coordinates and one temporal coordinate, and if we consider changing an object's spatial coordinates as motion through space, then why not consider changing the temporal coordinate as motion through time?Because that isn't what actually happens, and as (amateur?) physicists we want to make sure that our descriptions match reality.
I think it is a very important point, though, to consider that typically there is little relative "motion" along the timeline, and we only observe "now" so it is usually pretty useless to think about the time coordinates of objects. It is useful to think about the time coordinates of events though...It certainly is. Spacetime "works". But it isn't some actual thing that you can wander around at will. Ours is a world of space and motion. We can move around space, but time is just a cumulative measure of motion. The universe as we know it has been here for 13.8 billion light years. All that is, is a measure of how far light would have moved since the big bang.
Quote from: Ethos_
OK,......but would it then be accurate to say: "The advance of time has moved the location of our reality." .That doesn't sound accurate. The advance of time is fair enough, but moved the location of our reality doesn't sound right. The location of our reality is our little patch of this universe. And the reality is that we live in a world of space and motion.
Quote from: Ethos_
Because for time to be a dimension, it should progress in a linear fashionI don't think there's much issue with that, provided you appreciate that time is derived from the motion of things, and that there is no actual thing called time doing the progressing.
Quote from: Ethos_
The passage of these events is how we understand the change and as those changes take place, we experience them passing before us. It may be a little off mark to suggest motion through time but time is surely passing. And as it passes, it is moving us to a new reality.There no issue with events happening and changes taking place. But they don't actually pass. Nor does time. That's just a figure of speech. And whilst the present is not the same as the past, we haven't actually moved through time to go from one to the other. The place is the same place, things in it have been moving and changing, that's all.
Quote from: Ethos_
The passage of time is a collection of changing events in a region lying between the past and the future.There's no problem with the events or change, but there is no actual "region" lying between the past and the future.
Quote from: Ethos_
Just as the region lying between two points of width, length, or height are dimensions, so is the region lying between the past and future.Only they aren't the same. You can move around those spatial dimensions, and as you do your blood moves, your heart moves, electrochemical signals in your brain moves, things move in your clock, and the hands of your clock moves too. We then infer the time dimension from all this motion, but there is no real "region" between the past and present and future.
Quote from: Ethos_
And as such, a linear dimension as defined by a region lying between two points or locations. Time qualifies in that respect because each moment, each Planck moment places us in a different location in time. And because space and time can't be logically separated, a new location in space/time.The point to remember is that your measurements of space and time both employ the motion of light, such that when you move fast through space you measure time and space different to me. And note that you're moving through space, and so is the light.
Post by: PmbPhy on 15/02/2015 15:56:30
Is there no mathematician in this thread? [:(]Sure. How can I help you?
Post by: Bill S on 15/02/2015 21:29:03
Quote from: Pete
Sure. How can I help you?
Thanks, Pete, can I direct your attention to #78?
Post by: PmbPhy on 16/02/2015 00:33:26
Quote from: Bill S
Thanks, Pete, can I direct your attention to #78?Sure, buddy. It's simple. First we note how [ψ|2 is defined, i.e.
[ψ|2 = ψ*ψ
Therefore
|ψ1 + ψ2|2 = (ψ1 + ψ2)*(ψ1 + ψ2)
Note we note that the complex conjugate of a sum is the sum of the complex conjugates, i.e.
(ψ1 + ψ2)* = ψ1* + ψ2*
Now we multiply it all out
|ψ1 + ψ2|2 = (ψ1 + ψ2 )*(ψ1 + ψ2)
= (ψ1* + ψ2*)(ψ1 + ψ2)
= ψ1*ψ1 + ψ1*ψ2 + ψ2*ψ1 + ψ2*ψ2
= |ψ1|2 + |ψ2|2 + (ψ1*ψ2 + ψ2*ψ1)
Therefore
|ψ1 + ψ2|2 = |ψ1|2 + |ψ2|2 + (ψ1*ψ2 + ψ2*ψ1)
Simple, huh? :)
Post by: PmbPhy on 16/02/2015 00:39:52
Quote from: JohnDuffield
The point to remember is that your measurements of space and time both employ the motion of light, such that when you move fast through space you measure time and space different to me. And note that you're moving through space, and so is the light.Wrong yet again. While measurements of events in spacetime can be done using light it's not a necessity.
Post by: Bill S on 16/02/2015 17:19:57
Quote from: Pete
Simple, huh? :)
If you say so!
I was puzzled by the vertical lines which seem to be used as though they were brackets. I understand that they imply the absolute value of the figure they enclose. The nearest I could come to understanding the absolute value of a number was that it signifies the distance of that number from zero. I’m hard pressed to think of a calculation in which this distinction between value and absolute value might be significant.
3 = 1+1+1
|3| = 0+1+1+1 ?????
Post by: JohnDuffield on 16/02/2015 21:48:43
Wrong yet again. While measurements of events in spacetime can be done using light it's not a necessity.We use radar to measure distances, and our most accurate clocks are optical clocks. If you want to use a ruler and mechanical clock that's fine by me, but you will note that both consist of matter that has an electromagnetic nature. Hence the wiki article on gravitational time dilation referred to light and matter sharing the same essence. You can still see this on this A-level page (http://en.wikibooks.org/wiki/A-level_Physics/Cosmology/Relativity#Important_things_to_stress): "...electromagnetic radiation and matter may be equally affected, since they are made of the same essence...
Quote from: Bill S
If you say so!As you have doubtless learned already, on its own, maths doesn't really help with this sort of thing. What really helps, is experiment. That's not to say maths is futile. The refractive index in electron optics and the principles of dynamics (http://iopscience.iop.org/0370-1301/62/1/303) by Ehrenberg and Siday was heavy on maths. It dates from 1949, and it predicted what we nowadays call the Aharanov-Bohm effect. The electron wave isn't just something you can diffract. You can refract electrons too. Here's a picture from the paper, which I'm afraid is behind a paywall:
Post by: PmbPhy on 17/02/2015 15:10:28
It's important when the numbers are complex like the wave function. E.g. try as an exampleQuote from: PeteSimple, huh? :)
If you say so!
I was puzzled by the vertical lines which seem to be used as though they were brackets. I understand that they imply the absolute value of the figure they enclose. The nearest I could come to understanding the absolute value of a number was that it signifies the distance of that number from zero. I’m hard pressed to think of a calculation in which this distinction between value and absolute value might be significant.
3 = 1+1+1
|3| = 0+1+1+1 ?????
z = 1 + i
Post by: evan_au on 17/02/2015 20:42:42
Physicists explain the very high efficiency of photon capture and electron processing by suggesting that the chlorophyl molecule offers multiple paths for electron transfer, and the photoelectron effectively explores all of them as a delocalised coherent wavicle. This led to the (serious) field of quantum biology (http://en.wikipedia.org/wiki/Quantum_biology).
Post by: yor_on on 17/02/2015 21:34:51
Post by: jeffreyH on 17/02/2015 23:26:54
Think of e=mc^2 or
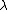 = h/p.
= h/p.
Post by: PmbPhy on 18/02/2015 08:08:37
One domain where an electron seems to be in multiple places at the same time is in photosynthesis.That's not the same thing as being in two places at once since at no time does any photon ever measured to be at more than one place. What you're talking about is Feynman's Explore all paths approach to the path integral formulation of quantum mechanics.
Physicists explain the very high efficiency of photon capture and electron processing by suggesting that the chlorophyl molecule offers multiple paths for electron transfer, and the photoelectron effectively explores all of them as a delocalised coherent wavicle. This led to the (serious) field of quantum biology (http://en.wikipedia.org/wiki/Quantum_biology).
http://en.wikipedia.org/wiki/Path_integral_formulation#Feynman.27s_interpretation
Post by: Bill S on 18/02/2015 11:59:57
Quote from: Pete
That's not the same thing as being in two places at once since at no time does any photon ever measured to be at more than one place.
Would it be right to say that the Path Integral Formulation maintains that the quon explores all paths, but a measurement would have to be made to establish the position of the quon at any given time?
Post by: Bill S on 18/02/2015 12:07:24
Quote from: Pete
try as an example
z = 1 + i
I would, if I had any idea what could be done with it. [:-\]
Post by: JohnDuffield on 18/02/2015 14:08:04
Would it be right to say that the Path Integral Formulation maintains that the quon explores all paths, but a measurement would have to be made to establish the position of the quon at any given time?No. That's old hat. A photon has its E=hc/λ wavelength, it doesn't have a localised pointlike position, just like a seismic wave doesn't have a localised pointlike position. See the physicsworld article In Praise of Weakness (http://www.physics.utoronto.ca/~aephraim/PWMar13steinberg-final.pdf). This describes how Aephraim Steinberg et al used "weak measurement" to map the path of a photon going though both slits of Young's double slit experiment:
(https://www.thenakedscientists.com/forum/proxy.php?request=http%3A%2F%2Fwww.physics.utoronto.ca%2F%7Eaephraim%2Fpics%2F3D.jpg&hash=0b5bb35528c03d2be8235f9b697c023e)
Its path is not some line, just as the path of a seismic wave isn't some line. However when you use strong measurement to detect the photon, it's like detecting a seismic wave with a pointy stick the size of a mountain range. It gets totally absorbed by the stick, and you think the location of the photon was at the point of the stick. Check out the optical Fourier transform, it's maybe something like. Something that isn't pointlike, looks pointlike:
(https://www.thenakedscientists.com/forum/proxy.php?request=http%3A%2F%2Fcns-alumni.bu.edu%2F%7Eslehar%2Ffourier%2Ffourier3.gif&hash=780cc0ec017e65e77263550fdf39e15b)
http://cns-alumni.bu.edu/~slehar/fourier/fourier.html
Post by: jeffreyH on 19/02/2015 00:02:54
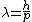 .
.NOTE: You need linear momentum here since de Broglie substituted velocity for c in deriving the general wave equation.
Post by: PmbPhy on 19/02/2015 16:52:40
Find the magnitude. That's done by using the expressionQuote from: Petetry as an example
z = 1 + i
I would, if I had any idea what could be done with it. [:-\]
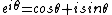
Then any complex number C can be represented as
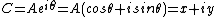
where

The magnitude of C is defined by
Post by: PmbPhy on 19/02/2015 16:56:42
Quote from: Bill S
Would it be right to say that the Path Integral Formulation maintains that the quon explores all paths, but a measurement would have to be made to establish the position of the quon at any given time?What's a "quon"? If you're talking about a particle then yes, that's true.
Quote from: JohnDuffield
No. That's old hat.In no way is the Path Integral Formulation of quantum mechanics "old hat."
Post by: Bill S on 19/02/2015 17:40:21
Quote from: Pete
What's a "quon"?
http://en.wikipedia.org/wiki/Quon
Sensu Nick Herbert.
Post by: PmbPhy on 19/02/2015 20:17:04
Interesting. I call them particles myself.Quote from: PeteWhat's a "quon"?
http://en.wikipedia.org/wiki/Quon
Sensu Nick Herbert.
Post by: JohnDuffield on 19/02/2015 22:21:18
In no way is the Path Integral Formulation of quantum mechanics "old hat".OK, let me restate: the notion that a photon is some point-particle thing with a discrete location is garbage.
Jeffrey: your point noted.
Bill: if you've read that book (http://en.wikipedia.org/wiki/Quantum_Reality#Eight_interpretations) I recommend you also watch this program (http://en.wikipedia.org/wiki/Tim_Shaw_(presenter)#None_of_the_Above).