Post by: Roju on 16/01/2015 17:08:52
I have always thought that when you pour NaCl(s) (or any ionic compound) into water, the
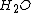 -atoms separate the Na+ and Cl- ions, dissolving the salt, and the saturation point is when there aren't any free
-atoms separate the Na+ and Cl- ions, dissolving the salt, and the saturation point is when there aren't any free 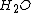 -atoms left to pull any more NaCl(s) apart, and at that point the excess salt remains solid at the bottom of the solution. Today i learned about the Common ion effect and i stumbled upon a problem.
-atoms left to pull any more NaCl(s) apart, and at that point the excess salt remains solid at the bottom of the solution. Today i learned about the Common ion effect and i stumbled upon a problem.With my understanding of the saturation point i dont fully grasp how if you have a saturated solution of AgCl and add NaCl(s), ignoring the change in volume, you can completely ignore the dissolved Na+-ions when calculating the solubility of AgCl in the new solution. Surely the Na+-ions will effect the solubility of AgCl if they occupy some of the limited
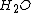 -atoms(which separate the salts into individual ions), as they are dissolved in the solution also.
-atoms(which separate the salts into individual ions), as they are dissolved in the solution also.If you only added Cl- ions i am completely onboard with the idea of the concentration of AgCl(s) in the solution going up, becuase there are too many free ions for the limited
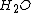 -atoms to manage and therefore you get precipitation.
-atoms to manage and therefore you get precipitation.Can someone tell me where im going wrong, and try to explain to me how it works?
What determines how soluable a salt is?
Thanks in advance.
Post by: chiralSPO on 16/01/2015 17:31:30
The real issue is the equilibrium between ionic solid and solvated ions--the ions have opposite charges, so they are attracted to one another. We have to account for the attraction of the ions to each other versus their attraction to the water. If it were just about having enough water molecules to surround the ions, how could you explain the difference in solubility between NaCl and AgCl?
As you increase the concentration of ions in solution, you are also decreasing the average distance between the ions, thereby increasing the effect they have on one another.
It easiest thinking about this in terms of solubility equilibrium constant (Ksp): where there is a (temperature-dependent) constant that represents the upper limit of the product of the ions concentrations in solution. For instance:
for AgCl at 25 °C, Ksp = [Ag+]x[Cl–] = 1.8x10–10 mol2/L2
so if a solution has a chloride concentration of 0.01 M, the maximum silver concentration would be 1.8x10–8
for PbCl2 at 25 °C, Ksp = [Pb+2]x[Cl–]x[Cl–] = [Pb+2]x[Cl–]2 = 1.7x10–5 mol3/L3
as we can see from this equation, even though lead chloride is more soluble than silver chloride, but it is more sensitive to chloride concentration because the chloride concentration is squared.
please look up Ksp online or in a general chemistry textbook for a more detailed explanation.
Post by: Roju on 16/01/2015 17:47:24
Aha. Good explaination, really cleared up all my questions, I allready know about Ksp, it was just my understanding of what a Saturated solution actually meant that got me confused. Many thanks!
The real issue is the equilibrium between ionic solid and solvated ions--the ions have opposite charges, so they are attracted to one another. We have to account for the attraction of the ions to each other versus their attraction to the water. If it were just about having enough water molecules to surround the ions, how could you explain the difference in solubility between NaCl and AgCl?
As you increase the concentration of ions in solution, you are also decreasing the average distance between the ions, thereby increasing the effect they have on one another.