Post by: JDPower on 08/08/2012 01:23:57
Post by: CliffordK on 08/08/2012 06:14:11
I'm seeing the diameter of a carbon atom (in meters) is about 1.52 × 10-10m ( 0.000 000 000 154 m) (http://answers.yahoo.com/question/index?qid=20080304094542AAa03zK)
And, here is a calculation for the volume of the human body (in meters), at about 0.07 m3 for a 70 kg person. (http://wiki.answers.com/Q/What_is_the_average_volume_space_of_the_human_body)
Hmmm
So:
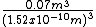 gives the approximate number of generic atoms.
gives the approximate number of generic atoms.[Alternatively, you might choose to calculate the number of moles and atoms in the body using Avagadro's number, 6.02214 ×1023. You could also estimate for the major constituents, carbon, oxygen, hydrogen, nitrogen, etc.]
To convert that back to meters, multiply that back by the size of a carbon atom.
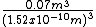 x
x 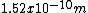
And you end up with:
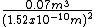 in meters.
in meters.And, I came up with about 3 × 1018m (3,000,000,000,000,000,000 m)
That is bigger than I can count in meters, but it comes out to about three quadrillion km.
A lightyear is about 9.4605284 × 1015 meters
So, you end up with about 300 lightyears.
While it doesn't quite reach across the Milky Way, it would reach a quite a number of stars.
Post by: JDPower on 08/08/2012 15:23:10
Post by: peptidechemist on 08/08/2012 22:41:51
I approached this based on the breakdown of the body into the individual elements. I have attached a table summarizing my calculations.
[attachment=post_tmp_27451_0][/attachment]
For a 70 kg person I calculated the weight of each element in the body. Then dividing by the molecular weight of each element you can determine the number of moles of each element in the body. Multiplying by Avogadro's number (6.022E23 atoms/mole) I calculated the number of atoms for each element.
Next, I multiplied the number of atoms by the atomic radius of each element. I used the single bond or covalent radius. This gave me the length of all the atoms lined up in meters, which I then converted to light years.
Post by: chris on 11/08/2012 21:16:33
Post by: peptidechemist on 16/08/2012 00:20:19
Using the compositional data from http://en.wikipedia.org/wiki/Composition_of_the_human_body#Composition_by_molecule_type I calculated the following
If you were to line up all the cells in your body they would stretch about 500,000 km which is a little further than the distance between the earth and the moon.
10^13 cells in the human body * 50 um (average size of a human cell) = 500,000,000 meters
If you were to take all the protein in the human body and line it up as a single elongated chain it would stretch about 2.5 light years, or if it formed a single alpha helix it would be roughly 1 light year long.
Humans are roughly 20% protein or around 12 kg on average.
(12,000 g protein) * (The average amino acid is 1 mol / 110 grams) * 6.022*10^23 amino acids / mol =6.57*10^25 amino acids.
The length of an amino acid is roughly 0.36 nm (distance between consecutive alpha carbons), so
6.57*10^25 amino acids * 0.36 nm = 2.4*10^16 meters = 2.5 light years
In an alpha helix each amino acid will increase the length of the helix by about 0.15 nm, so
6.57*10^25 amino acids * 0.15 nm = 9.85*10^15 meters = 1 light year.
If you were to put all of your DNA in a straight line it would be 20-30 billion km long or about 130-200 AU (The AU is the distance between the earth and the sun).
10^13 cells * 2-3 meters of DNA per cell = 20-30 billion km
If you were to line up all of the RNA in your body it would stretch 200-300 billion km, which is 1,300-2,000 AU, or roughly 7-11 light days.
Humans are roughly 0.1% DNA, but about 1% DNA and since DNA and RNA are similar your RNA would stretch 10 times further than your DNA
Post by: Antemortem on 18/07/2016 07:43:26
61.15% Oxygen 16g/mol - diameter 120x10^-12m
23% Carbon 12g/mol - diameter 140x10^-12m
10% Hydrogen 1g/mol - diameter 50x10^-12m
2.6% Nitrogen 14g/mol - diameter 130x10^-12m
1.4% Calcium 40g/mol - diameter 360x10^-12m
1,1% Phosphorus 30g/mol - diameter 200x10^-12m
0.2% Sulfur 32g/mol - diameter 200x10^-12m
0.2% Potassium 39g/mol - diameter 440x10^-12m
0.14% Sodium 22g/mol - diameter 360x10^-12m
0.12% Chlorine 35g/mol - diameter 200x10^-12m
For each element, the distance created by aligning each atom equals:
L=(Na*m*d)/M
Na being the Avogadro constant, m the mass in grams, d the diameter in meters, M in g/mol
So, for a 75kg human being, if we were to line up every single atom, the final distance would equal D=∑L=5.76E17 meters, that is to say roughly 3,850,267 times the distance between the Earth and the Sun, or 61 light years.
So if we stretch out far enough, we can reach beyond the Mensa constellation (Pi, Mensae, 59.4 light years away) to the Capricornius constellation (61.1 light years away).
Hopefully this will actually be useful at some point (if ever the universe becomes one-dimensional...)
Post by: syhprum on 18/07/2016 09:20:23