HIV Complex problem solved
Interview with
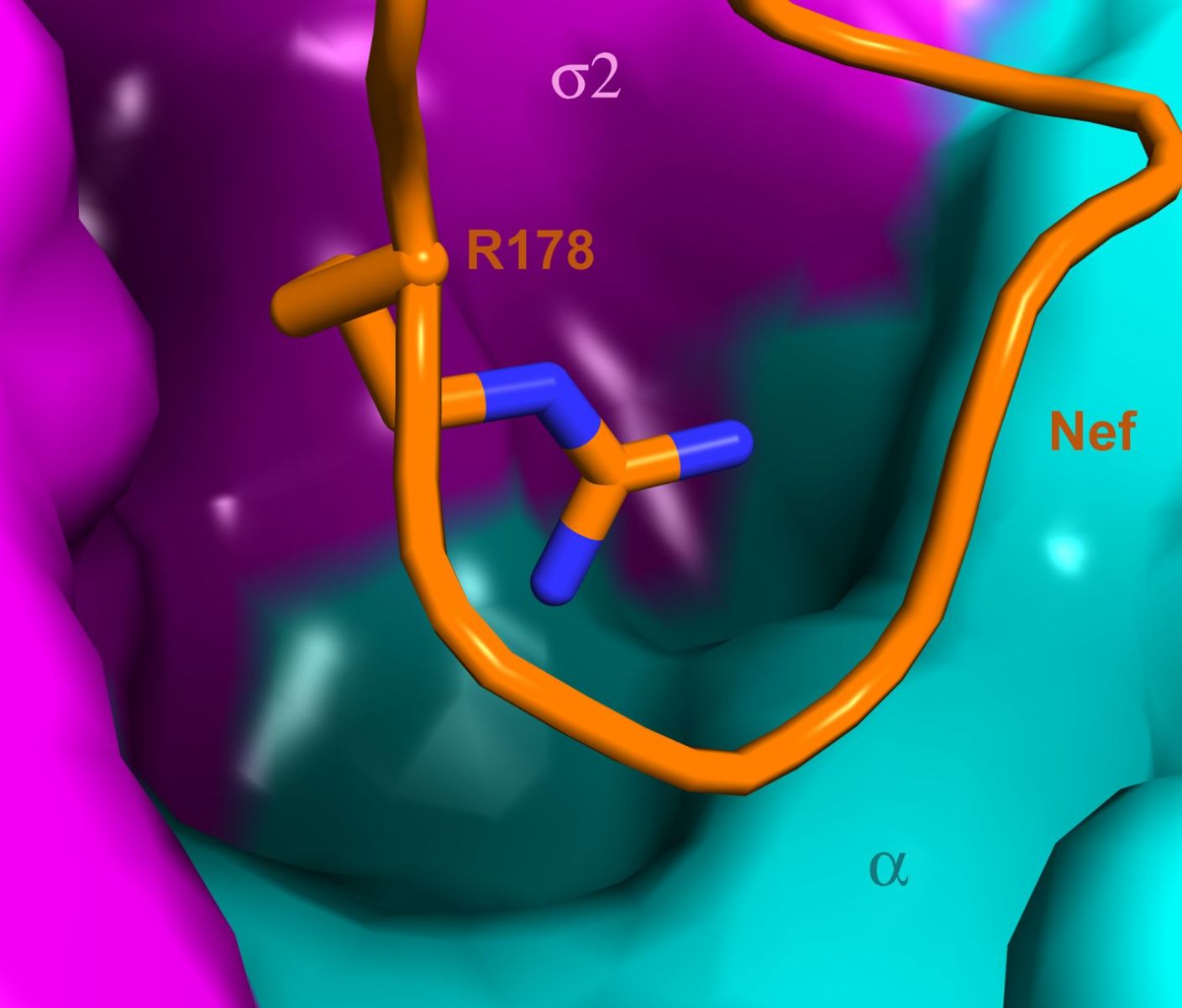
Researchers have worked out the structure of a protein complex that is involved in the destruction of T helper cells by HIV. We spoke to Jim Hurley from the University of California...
Jim - One of the interesting aspects to this whole class of cellular hijacking events is that it creates a vulnerability for the virus. So HIV has three enzymes - the reverse transcriptase, the integrase, and the protease, and these three enzymes are the targets for all of the approved drugs that are currently used to control AIDS, and do so quite effectively. However, there is an issue that the virus mutates rapidly and eventually, acquires resistance to these. So, we always need other lines of attack. And the human proteins obviously aren't evolving rapidly. Only the viral proteins are mutating. So if we can find places on human proteins that are used by the virus, but that might not be important for ordinary cellular functions, those would make excellent targets that might not be subject to this sort of rapid drug resistance. Now because we know that this process of downregulation of CD4 by NEF is so important for infectivity, this seemed like one of the excellent places to start.
Chris - So how did you go into the cell and ask where the NEF protein is acting in order to bring down the CD4, to rob the cell surface of this important molecule?
Jim - This information was pieced together over about two decades by many laboratories. A key insight that led to the current study came from the lab of my colleague, Juan Bonifacino, at the NIH. And what he did was carry out a screen in the cells derived from the fruit fly, of all things. The fruit fly isn't normally infected by HIV, but he created a clever screen in order to deduce which cellular proteins were most important for downregulating CD4s and discovered that only a handful of proteins worked with NEF and were absolutely essential for the effect. These are called a clathrin, which is a vesicle coat protein, and AP-2. AP-2 is what's called an adaptor protein. So it links a cargo protein. A cargo protein is whatever it is that gets downregulated to clathrin, in order to create a coated vesicle, which will then internalise into the cell and eventually carry the substrate protein to its destruction. So, the highlight of that work was the discovery that AP-2, of all the potential adaptors involved was the key factor in linking NEF and CD4 to each other.
Chris - And is that the one that you went ahead to look at?
Jim - Right. So in the current study, we characterised the interaction biochemically. So we able to measure the affinity, but more importantly, we were able to obtain crystals of the complex of the part of AP-2 that binds with NEF and obtain an atomic structure. So now, we can see atom by atom, the details of this interaction with great precision.
Chris - Does this reveal the long sought after, sort of virus-specific interaction that might be a target for a drug to interrupt that process?
Jim - We're quite excited about it because NEF has a motif - a region within it that copies the interaction's normal cargo use - and now when we perturb those new interactions, we can cripple the ability of NEF to downregulate CD4 in model cells. So, the site looks quite promising.
Chris Smith - What about the big R? The resistance problem. Because if you put pressure on the virus by interrupting that motif, that interaction, how easy will it be for the virus to mutate to surmount that problem?
Jim - It's certainly a possibility. So, I don't think we can rule out that that the virus will find a way around it. What the virus won't be able to do is to mutate the AP-2 side of the interaction. So, we'll have to find a different surface on AP-2 to interact with.
- Previous Into the maize
- Next Why rats don’t rat on other rats









Comments
Add a comment