Post by: worlov on 26/01/2019 16:10:46
It is explicitly stated in the literature that classical electrodynamics can not explain the photoelectric effect. In my view, there is at least one possibility.
How do quasi-free electrons move in the metals? I imagine a following model. At room temperature, the conducting electrons revolve around the metal ions, but at the points of contact between the metal atoms they pass from one atom to another without or with relatively little resistance.
Statistically nothing changes in principle if we disregard the short-term movement of the electrons between the atoms. In this way, we get a simplified model of the metal, which consists of hydrogen-like atoms. For the alkali metals, ie the metals belonging to the same group as hydrogen, this may even be quite true.
Further, a hydrogen atom is a resonator (the resonance frequency is 1.42 GHz). Therefore, the system of metal ion and quasi-free electron should also behave as a resonator. This raises the question of whether there is a relationship between the natural frequencies of such resonators and the corresponding cutoff frequencies of the photo effect.
Has this research been done?
Post by: Bored chemist on 26/01/2019 18:22:55
Statistically nothing changes in principle if we disregard the short-term movement of the electrons between the atoms.What changes is that you get a model which is wrong.
The sort of experiment you are talking about is done regularly.
https://en.wikipedia.org/wiki/Photoemission_spectroscopy
You also rather miss the point that those frequencies, such as the 1.42 GHz hydrogen line are the results of quantum effects.
Post by: worlov on 26/01/2019 20:27:07
Quote from: Bored chemist
What changes is that you get a model which is wrong.The models are very common in physics. They allow to make the estimates... And I think my model is plausible. The conducting electrons will not fly through the atoms, they will certainly move at the edges of atoms (figure below).
Quote from: Bored chemist
The sort of experiment you are talking about is done regularly.I mean purely classic approach. So I understand correctly that no one has thought about it yet.
Post by: Bored chemist on 26/01/2019 21:15:03
they will certainly move at the edges of atomsNo
The uncertainty principle prevents that.
The models are very common in physics.Models that work are very common.
Post by: alancalverd on 27/01/2019 00:04:27
Why do people persist with classical physics? Chemists stopped talking about caloric and phlogiston before BC was born. Quantum mechanics and relativity are older than the aeroplane. Nobody goes on holiday in a stagecoach, so why do they mess about with mathematical models that don't work?
Post by: worlov on 27/01/2019 09:05:11
Quote from: Bored chemist
The uncertainty principle prevents that.The conducting electrons will not fly through the atoms, otherwise it would then come to the generation of X-rays.
Post by: worlov on 27/01/2019 09:24:20
Quote from: alancalverd
Why do people persist with classical physics? ... Nobody goes on holiday in a stagecoach, so why do they mess about with mathematical models that don't work?And why should any alternative idea be rejected immediately? Are not you curious? Maybe something was overlooked 100 years ago.
Post by: Bored chemist on 27/01/2019 09:43:27
According to classical physics, they should be doing that.Quote from: Bored chemistThe uncertainty principle prevents that.The conducting electrons will not fly through the atoms, otherwise it would then come to the generation of X-rays.
That's why we know classical physics is wrong.
Post by: Bored chemist on 27/01/2019 09:47:18
Not much photoelectricity occurs with photon frequencies below 1013 Hz, a lot more than 1.42 GHz.Almost all the engineering/ design/ physics I have actually done (as opposed to reading about) was classical physics.
Why do people persist with classical physics? Chemists stopped talking about caloric and phlogiston before BC was born. Quantum mechanics and relativity are older than the aeroplane. Nobody goes on holiday in a stagecoach, so why do they mess about with mathematical models that don't work?
Similarly, sending a man to the Moon didn't need to take account of relativity, but adding the relativistic terms would have cluttered up their very limited computational power.
So, people often use classical physics because, at low speeds, for big things, it works well enough.
Post by: worlov on 27/01/2019 11:22:34
Quote from: Bored chemist
According to classical physics, they should be doing that.Why? The conducting electron is the outermost electron of the atom. The other electrons within the atom will repel the outer electron. Yes, the positively charged metal ion generally attracts the outer electron, but the other electrons form the barriers on the way to the nucleus.
Post by: Bored chemist on 27/01/2019 12:45:19
Why? The conducting electron is the outermost electron of the atom.And it should "fall" into the nucleus, emitting X Rays as it goes.
Post by: jeffreyH on 27/01/2019 15:25:21
Post by: alancalverd on 27/01/2019 18:54:43
Post by: evan_au on 27/01/2019 20:02:46
Quote from: worlov
a hydrogen atom is a resonator (the resonance frequency is 1.42 GHz)You are describing the 21cm "Hydrogen line", used by radio astronomers to map the concentration of neutral hydrogen gas in the galaxy and across the universe.
https://en.wikipedia.org/wiki/Hydrogen_line
Rather than being a natural, strong resonator, this is an incredibly weak "forbidden" resonance, which is what makes it so useful to astronomers.
- Hydrogen is so abundant in the universe that if it were a strong resonance, radio astronomers could not see out of the Solar System, because there is so much hydrogen in the Solar wind.
- In fact, this transition is extremely rare, and it takes a massive cloud of gas (thousands of times more massive than the Sun) to produce a measurable signal in radio telescopes.
- Natural emission of this hydrogen line is considered impossible to detect in the lab, but it can be artificially triggered
- On the other hand, the photoelectric effect can be readily detected in the lab - but with energies billions of times greater (UV photons instead of microwave photons)
Quote from: worlov
I imagine a following model. At room temperature, the conducting electrons revolve around the metal ions, but at the points of contact between the metal atoms they pass from one atom to another without or with relatively little resistanceWhat you are describing is similar to the Fermi surface, which describes the permissible momentum of electrons in the conduction band of a metal.
- If you join a few of the units together, It starts to look like a Swiss cheese
- The inner electrons are strongly attached to the nucleus, and don't participate in electrical conductivity
See: https://www.google.com/search?tbm=isch&q=fermi+surface+aluminum
https://en.wikipedia.org/wiki/Fermi_surface
Quote
the system of metal ion and quasi-free electron should also behave as a resonator. Has this research been done?Yes, this is how the Fermi surface was initially mapped. It is typically done at very low temperatures, in a very strong superconducting magnet.
A more recent method is to bombard the metal with positrons.
Quote
For the alkali metals, ie the metals belonging to the same group as hydrogen, this may even be quite true.Hydrogen as we have it on earth is a non-conductor.
- However, at the enormous pressures at the core of Jupiter, physicists believe that Hydrogen enters a metallic state, producing Jupiter's considerable magnetic field.
- Recently, scientists made a (somewhat controversial) claim to have produced metallic hydrogen in the lab (under enormous pressures in a diamond anvil). The most visible change was that it was no longer transparent.
- So the behavior of non-conductive hydrogen is totally different from metallic hydrogen.
Quote
Statistically nothing changes in principle if we disregard the short-term movement of the electrons between the atoms. In this way, we get a simplified model of the metal, which consists of hydrogen-like atoms.I am afraid that conduction between atoms totally changes the behavior of a substance when irradiated by electromagnetic waves.
- For non-conductive materials, electromagnetic waves tend to pass right through (with a bit of refraction, as the speed of light is lower in matter than it is in a vacuum)
- For highly conductive materials like metals, electromagnetic waves cause movement of the surface electrons, inducing a current in the surface of the metal which is opposite and equal to the external field (by Lenz's Law). This cancels the incoming wave, and generates an outgoing wave where the angles follow the law of reflection that you learnt in High School.
Quote
the corresponding cutoff frequencies of the photo effectThe cutoff frequency of the photoelectric effect is determined by the Work Function of the metal.
It is closely related to the Fermi energy of the electrons.
It is not closely related to the Hydrogen Line.
See: https://en.wikipedia.org/wiki/Work_function
Post by: worlov on 28/01/2019 09:57:59
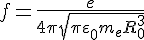
R0 is the atomic radius. For different elements there is the table (last column)
http://en.wikipedia.org/wiki/Atomic_radii_of_the_elements_(data_page)
When I set the values, I get the resonance frequencies close to the cutoff frequencies of the photo effect. There is supposed to be a connection.
Post by: worlov on 28/01/2019 18:55:43
Post by: Bored chemist on 28/01/2019 19:24:41
Furthermore, the frequencies correlate within the groups and the periods.So do the weights, but that doesn't mean the effects are dominated by gravity.
the formula for the resonance frequency isWhat resonance is that?
Post by: evan_au on 28/01/2019 20:14:30
However, the 21cm/1.4GHz atomic Hydrogen line occurs because there is:
- Just 1 unpaired electron, which acts like a tiny magnet
- Just 1 unpaired proton, which acts like a tiny magnet
- It is the tiny interaction between these two tiny magnets (parallel/anti-parallel) which produce the tiny energy transition of 5.8 μeV.
However, when there are:
- 2 electrons in the S orbital, their spins cancel each other out, eliminating Hydrogen's tiny magnetic field from electrons
- An even number of protons in the nucleus, their spins cancel each other out, eliminating Hydrogen's tiny magnetic field from the nucleus
- This also eliminates the tiny energy transition which generates the 21cm Hydrogen line
- And the quoted resonance formula becomes irrelevant
Also, when isolated atoms form up into molecules, the electrons tend to pair up so that their spins cancel, eliminating this supposed resonance. And the photoelectric effect can be seen with molecules as well as metals.
Please identify the source of "the formula for the resonance frequency" - I am sure there are a lot of caveats there that you have ignored!
I would be especially interested to see the correlation in Group 4 (Carbon to Lead), which makes the transition from non-metal to metal, and involves significant changes in the Fermi Surface and Fermi Energy.
The correlation in the graphs may have more to do with the dependence of the Work function on the atomic radius.
- ie correlation does not show causation
Post by: worlov on 29/01/2019 07:11:11
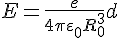
d - the shift of the electron ball relative to the metal ion ball. This field strength acts on the charge of the electron, resulting in the force that tries to bring the electron back to its rest position
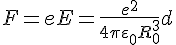
As we can see, retroactive force is directly proportional to the shift. So we're getting to Hooke's Law. Therefore, we have for the spring constant
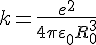
Then the resonance frequency
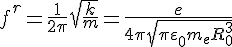
Logically, when the frequency of the external electric field coincides with the natural frequency of the conducting electrons, the resonential phenomenon occurs and the conducting electron is thrown out of the atom and finally out of the Metall.
Post by: evan_au on 29/01/2019 09:32:11
Quote
Logically, when the frequency of the external electric field coincides with the natural frequency of the conducting electrons, the resonential phenomenon occurs and the conducting electron is thrown out of the atom and finally out of the Metal.The energy of the Hydrogen 1.4GHz resonance is 5.8 μeV.
The energy of the photoelectric work function is typically around 3-5 eV.
It is not logical to think that bombarding an electron with nx5.8 μeV could trigger an event requiring an energy a million times greater.
One of the principals of the photoelectric effect is that it is an "all-or-nothing" effect.
- One photon kicks out an electron, or it does not
- Atoms don't accumulate energy from radiation until they reach a threshold and then spit out an electron
- The whole idea of a resonance is that it works for some frequencies, and not others
- The "right" energies are in the Ultraviolet part of the spectrum
- Energies in the microwave band are irrelevant - by 6 orders of magnitude!
See: ]List of Work functions (https://en.wikipedia.org/wiki/Work_function#Work_functions_of_elements[11)
Post by: worlov on 29/01/2019 13:46:56
The energy of the Hydrogen 1.4GHz resonance is 5.8 μeV.The energy of the photoelectric work function is typically around 3-5 eV.I mentioned Hydrogen 1.4 GHz resonance as an example. The whole treatment concerns the solids. And I get the frequencies that are in the order of the work function and even more. I use a very simple model. Considering the interaction with the neighboring atoms, the frequencies will sink and then even fully agree with the experimental results. Qualitatively I see no problem for the resonance hypothesis of the photo effect. A photon counter counts the photons less often at low light intensity. And so it would be expected in a resonance phenomenon: it takes more time to spin up the electron with less light intensity for the necessary kinetic energy.
Post by: alancalverd on 29/01/2019 16:47:43
You need to distinguish between photon energy and beam intensity. The photoelectric effect is observed with single photons above the work function energy. A photon counter counts photons less often at low intensity because they are arriving less often.
Post by: Bored chemist on 29/01/2019 16:56:10
The electron is a negatively uniformly charged ball and the metal ion is a positively uniformly charged ball. The two are about the same size
That is probably the most wildly wrong assertion ever made.
"According to modern understanding, the electron is a point particle with a point charge and no spatial extent. Attempts to model the electron as a non-point particle have been described as ill-conceived and counter-pedagogic" (from WIKI)
https://en.wikipedia.org/wiki/Classical_electron_radius
So, the metal ion has a finite size and the electron is infinitely smaller.
Yet you say they are about the same size.
Which makes you infinitely wrong.
Post by: evan_au on 29/01/2019 20:40:48
Quote from: worlov
we're getting to Hooke's Law. Therefore, we have for the spring constant...Quantum events are not like a classical analogue spring (which follows Hooke's Law).
- An analogue spring can collect energy near its resonance frequency, increasing amplitude over time until something breaks.
- Quantum interactions only take on specific energies;
- if an incoming photon has this specific energy, it can interact
- if an incoming photon has insufficient energy, it will pass through
- if an incoming photon has excess energy, it will often interact, but the excess energy has to go somewhere
In the case of the photoelectric effect, this specific energy is the work function.
- In case of excess energy, it goes into the velocity of the emitted electron. This velocity is not a quantum thing, but is a classical velocity which can take on all values of energy.
- Hence, the photoelectric effect does not exhibit a resonance, but a threshold
- Above this work function threshold, the emitted electron shows the relationship that photon energy is proportional to frequency (via Planck's constant)
Post by: worlov on 30/01/2019 07:47:26
A photon counter counts photons less often at low intensity because they are arriving less often.Yes, it can be explained this way and that. And the sensitivity curves of the photon detectors speak also for the resonance. They look like the damped resonance curve (figure below).
So, the metal ion has a finite size and the electron is infinitely smaller.Yet you say they are about the same size.The metal ion is immobile. The electron, on the other hand, is very fast. In its chaotic movement around the metal ion, it blurs into a sphere.
Post by: esquire on 30/01/2019 18:17:14
en.wikipedia.org/wiki/Magnetohydrodynamic_drive
en.wikipedia.org/wiki/Variable_Specific_Impulse_Magnetoplasma_Rocket
Post by: alancalverd on 30/01/2019 18:34:04
A photon counter counts photons less often at low intensity because they are arriving less often.Yes, it can be explained this way and that. And the sensitivity curves of the photon detectors speak also for the resonance. They look like the damped resonance curve (figure below).
No. There is no explanatory and predictive classical mechanism for the photoelectric effect, which (along with the ultraviolet catatstrophe) is why we use quantum physics that also predicts a whole lot more and doesn't involve absurdities like orbiting electrons.
I have a moustache and white hair. On a windy day I could look like Einstein, but it wouldn't make me a genius.
Post by: Bored chemist on 30/01/2019 19:22:40
Yes, it can be explained this way and that. And the sensitivity curves of the photon detectors speak also for the resonance. They look like the damped resonance curve (figure below).Those are not the same thing.
For what it's worth, absorption spectra generally look rather like damped resonances- because the maths looks pretty similar, but this is the model they use, rather than a ball on a spring
https://en.wikipedia.org/wiki/Perturbation_theory_(quantum_mechanics)#Time-dependent_perturbation_theory
A quick search of the internet lead me to the magnetohydrodynamic drive. As I mentioned above, a very similar theory and the same keywords/buzzwords are employed to explain your photoelectric effect as resonance effect, with practical variation implementedThose really have practically nothing in common.
Post by: Bored chemist on 30/01/2019 19:27:43
The metal ion is immobile. The electron, on the other hand, is very fast. In its chaotic movement around the metal ion, it blurs into a sphere.A chaotically moving charged particle like an electron would emit radiation and stop moving.
Also, that's not what you said earlier.
You said it was the same size as an atom, and that's infinitely wrong.
Post by: korosten on 30/01/2021 15:03:50
I just stumbled upon your idea and I think it makes a lot of sense - I recently had a similar discussion on another forum and I was wondering about the same thing.
If it were due to resonance, then one would expect that a change in intensity would merely change the probability of an event, which is indeed observed (lower intensity would mean that on average it takes longer for an event to occur). I would be interested to see if there is any correlation between intensity and the time it takes for an electron to be emitted.
There have been a few papers recently that determined the amount of time it takes for different atoms, which is in the order of attoseconds (so it is not "immediate" as it is sometimes claimed).
Here are some references that might be relevant:
"Time-delayed photoelectric effect "
"....We describe here photoelectric emission (PE) experiments using very low-intensity nanosecond light pulses with energies near the PE threshold. Signal correlated, time-delayed pulses of emitted electrons were observed for single light pulses incident on a photosensitive material."
(I am not able to post links, so here are just the titles)
"Controlling the Photoelectric Effect in the TimeDomain"
"Photoemission and photoionization time delays and rates"
Maybe this one: "Absolute timing of the photoelectric effect" ?
It seems to me, if resonance is indeed the cause then we would expect a slight delay for ultra low intensities. I am not sure if this experiment has been done yet or not and how to compute the expected delay? Does anyone know?
Best wishes,
Chantal