Post by: timey on 29/10/2016 01:57:13
https://en.m.wikipedia.org/wiki/Ultraviolet_catastrophe
Frequency is a count of how many wave cycles complete within the time scale of a standard second.
E=hf and Wavelength=hv
What I'm going to do is decrease the length of a second as energy increases.
So for this calculation, by replacing Wavelength=hv with Wavelength=hf, where frequency is the velocity of a second - we are now keeping the 'distance' of a wavelength constant and instead decreasing the length of a second that the cycles of the wave complete within...
Do the curves now match?
Post by: evan_au on 29/10/2016 02:53:07
However, the frequency of emitted or absorbed photons is determined by electron orbitals. Classical electromagnetism cannot explain why electrons only reside in certain energy levels, or why there is a "ground state" energy level.
To understand electron orbitals in an atom, you really need to understand Schroedinger's equation - and be able to solve it.
See: https://en.wikipedia.org/wiki/Schr%C3%B6dinger_equation
Post by: timey on 29/10/2016 03:22:26
Actually I'm a little unsure of the maths I've given. The idea of Wavelength=hv would be transposed to Secondlength, but on puzzling over it I think Secondlength would have to be Secondlength=f/h...
In any case the idea is that the length of a wavelength, or the distance that it travels remains constant, and that it is the length of a second that is decreasing as energy increases.
This concept ties in with the measurement of joules per 'standard' second and should eliminate the quantised nature of the data.
Post by: jeffreyH on 29/10/2016 11:37:27
Post by: timey on 29/10/2016 12:21:12
Planck tried everything to iron out the quantised nature of his data. By measuring joules per standard second and frequency as cycles per standard second, Planck has standardised time in the equation.
But time is not standard, it is variable, and if adding energy makes the frequency of wave cycles increase, why not try proportionally decreasing the length of a second for a faster second accordingly?
This should eliminate the quantised nature of the data!
There are no messing around with dimensions being conducted in this process. Indeed nature reflects this concept in that food left in a fridge will last longer than food that is not.
Post by: jeffreyH on 29/10/2016 12:30:05
Post by: jeffreyH on 29/10/2016 12:34:19
Post by: jeffreyH on 29/10/2016 12:37:02
Post by: timey on 29/10/2016 12:59:48
I do not understand why the cycles of the waves are dimensionless...
In fact it would seem to me that giving a wave cycle a dimension and stating the frequency of the cycles as time related would be mathematically possible...
Then just measure the addition of joules as per the variable seconds as denoted by increase in frequency, and the quantised nature of the data is eliminated.
Post by: jeffreyH on 29/10/2016 13:19:02
Post by: timey on 29/10/2016 13:38:39
...and adding joules to seconds that are decreased in length will alter the data curve.
As stands the wavelength has an associated length in 'distance' that is proportional to energy. All I suggest is that this length in 'distance' is transposed to being a length of 'time'.
Post by: jeffreyH on 29/10/2016 13:54:08
Post by: timey on 29/10/2016 14:08:02
I daresay the world of physics would be quite happy to consider a hypothetical model of quantised dark energy though, aye?
But you reckon that subtracting time from the length of a standard second for a linear data curve is a no, no...?
Post by: jeffreyH on 29/10/2016 14:17:52
https://en.m.wikipedia.org/wiki/Wien_approximation (https://en.m.wikipedia.org/wiki/Wien_approximation)
Post by: jeffreyH on 29/10/2016 14:23:49
If time is a variable, which we know it is, then assigning a wave function as time related is far less outlandish than assigning an unobserved and mechanically unaccounted for energy to propel everything in the universe outwards at an ever accelerating rate.
I daresay the world of physics would be quite happy to consider a quantised dark energy though, aye?
But you reckon that subtracting time from the length of a standard second for a linear data curve is a no, no...? Kg
Putting words into someone's mouth is a standard defence mechanism of those protecting an untenable position. An attempt to cast doubt to deflect attention away from a defect in logic. Just thought I would clarify that point.
Post by: timey on 29/10/2016 14:43:25
I did not put words in your mouth, it was a question...
Physics is quite happy to consider adding a hypothetical dimension of dark energy to support the continuation of current theory. I'm questioning the fact of you seeming to reckon that adding a hypothetical decrease in the length of a second to achieve a linear and classical data curve is a no, no - in the face of what current physics adds to maintain the premise of the current theory.
More to the point, does what I'm suggesting actually even achieve a linear and classical curve? This being the very first of my considerations...whether or not it's acceptable to achieve linear results in this way being the second.
Post by: jeffreyH on 29/10/2016 14:48:34
Post by: timey on 29/10/2016 15:50:34
A brief and oversimplified synopsis in my own words:
Planck added energy to a blackbody. As energy was added, the frequency that the resulting radiation was emitted at increased, but not in a linear fashion as the Rayleigh-Jean law predicted. In trying to make the data fit the predicted curve, Planck found that by splitting the input energy into lumps, there could be gaps between the frequency changes. Frequency changes that only occurred at certain energy levels and that the 6.whatever joules per second was the constant that emerged. ie: Planck's h constant.
Post by: jeffreyH on 29/10/2016 16:13:11
https://en.m.wikipedia.org/wiki/Max_Planck (https://en.m.wikipedia.org/wiki/Max_Planck)
Post by: timey on 29/10/2016 16:32:59
What am I looking for Jeff?
Are you saying my synopsis is wrong?
Post by: jeffreyH on 29/10/2016 16:39:13
Post by: timey on 29/10/2016 16:51:30
I'm simply suggesting a possibility that I've seen to do what Planck did not manage, despite his best efforts, and linearise the maths.
Post by: jeffreyH on 29/10/2016 17:01:35
http://mobile.nytimes.com/2010/06/13/books/review/Farmelo-t.html (http://mobile.nytimes.com/2010/06/13/books/review/Farmelo-t.html)
It doesn't impress me.
Post by: jeffreyH on 29/10/2016 17:07:18
https://en.m.wikipedia.org/wiki/Manjit_Kumar (https://en.m.wikipedia.org/wiki/Manjit_Kumar)
What work has he done in the field of theoretical or experimental physics? Quote mining is no substitute for background.
Post by: jeffreyH on 29/10/2016 17:09:55
Post by: timey on 29/10/2016 17:16:33
Are you saying my synopsis is wrong?
Post by: jeffreyH on 29/10/2016 19:13:38
Not in the slightest, and it was Planck who was quoted as saying that he had fudged the maths, I'm certainly not qualified to make such an assertion.
I'm simply suggesting a possibility that I've seen to do what Planck did not manage, despite his best efforts, and linearise the maths.
Can you provide either a link to this quote of Planck's or post details from your book. Otherwise how can I make a proper determination.
Post by: Colin2B on 29/10/2016 19:29:03
Can Planck's law curve be matched to Rayleigh-Jean's law curve like this?The Rayleigh curve does not peak so could not be matched.
…..
Do the curves now match?
If you change the time with frequency then the observed peak would be shifted and wouldn't occur in its observed position.
This concept ties in with the measurement of joules per 'standard' second and should eliminate the quantised nature of the data.Could you explain your thinking on this?
But time is not standard, it is variable,
Although time varies between reference frames and places of differing gravity, there is no indication that it varies within an inertial frame. Light of different frequencies doesn't show any sign of varying in speed.
Post by: jeffreyH on 29/10/2016 19:51:22
http://www.testandmeasurementtips.com/plancks-constant-and-discrete-energy-levels/ (http://www.testandmeasurementtips.com/plancks-constant-and-discrete-energy-levels/)
Is this what you are referring to? If so you have the wrong end of the stick entirely.
Post by: timey on 29/10/2016 19:56:17
There is evidence of time varying within the same reference frame. Food put in a fridge will last longer than food that is not. Cryogenics is a consideration also.
Post by: jeffreyH on 29/10/2016 20:22:16
Colin - I am suggesting that by calculating joules added as per a second that is becoming shorter as energy is added, that the quantised nature of calculating joules added as per standard second will be eliminated, and that the Planck data curve will no longer peak, but follow the Rayleigh-Jean law classical curve.
What the hell is that supposed to mean? Your grasp of terminology is appalling. Do us the courtesy of actually paying attention.
Quote
There is evidence of time varying within the same reference frame. Food put in a fridge will last longer than food that is not. Cryogenics is a consideration also.
Are you seriously suggesting that temperature affects time? It isn't a crime to be wrong or to admitting it.
Post by: Ethos_ on 29/10/2016 22:16:37
I have been following your posts on these speculations of yours for a considerable length of time now timey. And frankly, I've been considering your ideas and been inclined to give you every benefit of doubt taking into account your sincerity.
There is evidence of time varying within the same reference frame. Food put in a fridge will last longer than food that is not. Cryogenics is a consideration also.
I think you already know that I would be tickled to find evidence for the cyclical universe, and for this reason I've been secretly hoping that you could present such evidence for us all to ponder. Nevertheless, this last statement of yours has left me with my mouth literally hanging open in amazement.
Any confidence I may have had in the possibility that you may have been on to something new has completely evaporated. That you can equate a reduction in spoilage to a slowing of time is insulting to Louis Pasteur.
Post by: Colin2B on 29/10/2016 23:58:20
Colin - I am suggesting that by calculating joules added as per a second that is becoming shorter as energy is added, that the quantised nature of calculating joules added as per standard second will be eliminated, and that the Planck data curve will no longer peak, but follow the Rayleigh-Jean law classical curve.But the Rayleigh curve goes off the scale at higher frequencies, are you suggesting that energies in the uv zone are tending towards infinite?
Also tha Planck curve is a combination of the Rayleigh-Jean and Wien curves, Planck managed to link them sorting out the discontinuity. You need to show your calculations and for both curves..
There is evidence of time varying within the same reference frame. Food put in a fridge will last longer than food that is not. Cryogenics is a consideration also.Please show the evidence.
I have a great deal of sympathy with the view of gravitational time dilation being relative to free fall or a true zero gravity, but this is beginning to sound silly when you introduce cryogenics into time/frequency variations.
Don't go Box on us please.
Post by: timey on 30/10/2016 00:03:54
Colin - what on earth are you talking about pay attention? I already described earlier this thread my thoughts concerning joules being measured as per standard second. And reducing or extending the length of a second is not exactly new terminology. Unless you mean anything other, then please do the courtesy of explaining that which you are not comprehending.
Colin and Ethos - In case it has escaped your attention the cesium atomic clock must be cooled radically to a constant temperature. Any increase in temperature will increase its rate of time.
Ethos - I can appreciate that in a model as fully encompassing as mine it is easy to miss detail, but my model states that GR time dilation is a mass near mass related phenomenon and that all bodies of mass experience time differently including living bodies. Living bodies are not time affected by temperature changes because of internal coping mechanisms. Some non-living bodies decay slower at low temperatures and faster at high temperatures... Plants/trees grow faster in times of global warming, and slower during ice ages.
Cryogenics can theoretically suspend the aging of a body using temperature... I really don't see your problem with the analogy of different rates of time occurring in the same reference frame... although I can appreciate that people do have trouble already with grasping the concept of time being a variable within the remit of Special and General relativity. I know you are not one of these people but can recognise that as I am expanding the concept of variable time quite considerably, people may take a while to understand.
Post by: Colin2B on 30/10/2016 00:09:50
Post by: jeffreyH on 30/10/2016 00:14:05
Jeff - What does it matter about Planck saying that he'd fudged the maths. That's what he apparently said according to Manjit Kumar and I've no doubt it is cited. But so what? ... And the link you posted - are you saying that the text suggests that my synopsis is incorrect?
You made an issue of it or does your memory conveniently fail you?
Post by: timey on 30/10/2016 02:19:06
Jeff - What does it matter about Planck saying that he'd fudged the maths. That's what he apparently said according to Manjit Kumar and I've no doubt it is cited. But so what? ... And the link you posted - are you saying that the text suggests that my synopsis is incorrect?
You made an issue of it or does your memory conveniently fail you?
Your memory seems to fail you Jeff, as I distinctly made this comment that I read that Planck made in the context of explaining the means by which Planck discovered his h constant. He was trying to match his data to the classical curve, and described it later as fudging.
You still haven't answered if my synopsis is wrong!
Post by: timey on 30/10/2016 06:16:47
Forget it
Thank you for your input. Please forgive my impatience, but I am tempted to get my son to make a video of me trying to make posts on this broken phone. The screen constantly freezes up, or directs me to wrong page, and I'm uncertain about the future of my internet connection.
The question was concerning if decreasing the length of a standard second proportionally to increased energy as the Planck data curve diverges from the classical curve in the higher frequencies - or indeed adding to the length of a standard second proportionally to decreased energy where the Planck curve diverges from the classical curve in the lower frequencies - and if measuring joules per second added to the blackbody via these variable seconds as energy is added, would the Planck data be rendered linear?
The question is not concerning if doing so is appropriate, just if Planck's data could then be linear instead of quantised.
(It was my thought 2 years ago that I could explain my model quite simply as a cyclic universe that finds its beginning and end of cycles via the black hole phenomenon in a contracting universe that inflates via the superluminal jets of the last standing singular black hole of the previous cycle, and develops from a sea of particles into clumped mass on the contraction trajectory as particles are drawn to each other... and empty spaces are created where the particles once where. That this model is GR without the cosmological constant, and redresses Hubble's redshifts velocities as being gravitational time dilation related with the introduction of a time dilation for the gravity field of open space in relation to mass, that runs counter directional to GR gravitational time dilation in a gravitational field, and that a wave'length' is not actually distance related but time related. That GR gravitational time dilation is a mass near mass phenomenon due to mass being affected by gravity potential, and massless entities such as light are, by their lacking in mass, not affected by gravity potential energy and are travelling through a counter directional gravitational time dilation... and that lights wavelength lengthening in the weaker gravity field is due to travelling through reference frames of open space with time that is slower than a standard second. Stating that the phenomenon of time itself is a byproduct of energy and part and parcel of the mechanics of the universe. Adding a few details in about the viewing of variable times in relation to the Bekenstien Hawking conundrum concerning energy conservation law and second law thermodynamics, and explaining an alteration to the equivalence principle that ensures the speed of light is not exceeded...
In posting this descriotiin elsewhere on the forum, I had thought that someone here on the forum would be able to relate all these descriptions with each other and come up with more of a response than:
"those dimensions are meaningless"
Meaningless with respect to what?
However... here on physics board I am asking a quite simple mathematical question (for a mathematician) about a process that Planck attempted and failed to manage... This being could what I suggest be a mathematical means to linearise Planck's data.
Post by: evan_au on 30/10/2016 09:54:03
Quote from: timey
the cesium atomic clock must be cooled radically to a constant temperature. Any increase in temperature will increase its rate of time.The cesium atoms in a cesium fountain are cooled to a low temperature, but not for the reason you suggest.
- When atoms have a temperature above absolute zero, the atoms are all moving at different speeds, in different directions, and this introduces a random Doppler shift. This makes it hard to focus on the precise resonant frequency of the atom, because every atom has a different Doppler shift.
- The increased temperature increases the apparent width of the resonance frequency, but not the center frequency.
- According to your theory, if you halved the temperature, the rate of time would be halved, and this is not observed.
- Today's best cesium fountain clocks cool the cesium atoms to near absolute zero - according to your theory, these clocks would not work (or would be horribly inaccurate), because time would almost stop.
See: https://en.wikipedia.org/wiki/Atomic_clock
Cesium clocks are not the only kind of clocks in the universe.
- The first atomic clocks were Hydrogen masers, that operated at room temperature.
- Hydrogen atoms in the Sun emit light at particular frequencies, depending on the electron's energy level before and after the photon is emitted.
- The Hydrogen atoms visible on the Sun can have temperatures from around 5500K to far higher in areas subject to magnetic heating.
- And yet the frequency of these photons is what Schroedinger equation predicts for room temperature.
- With lasers, we can pump room-temperature Hydrogen into any energy level we like, and it matches the frequencies emitted by the Sun, at much higher temperatures.
Timey, I have got cold feet about your new theory. I think it will take forever for you to come to any conclusions.
Post by: jeffreyH on 30/10/2016 09:55:10
Post by: Bored chemist on 30/10/2016 10:44:25
Can Planck's law curve be matched to Rayleigh-Jean's law curve like this?
https://en.m.wikipedia.org/wiki/Ultraviolet_catastrophe
Frequency is a count of how many wave cycles complete within the time scale of a standard second.
E=hf and Wavelength=hv
What I'm going to do is decrease the length of a second as energy increases.
So for this calculation, by replacing Wavelength=hv with Wavelength=hf, where frequency is the velocity of a second - we are now keeping the 'distance' of a wavelength constant and instead decreasing the length of a second that the cycles of the wave complete within...
Do the curves now match?
Before we can make any meaningful progress you need to explain what you mean by "What I'm going to do is decrease the length of a second as energy increases."
Time is a real thing, it exists; you can't change it arbitrarily.
Post by: Colin2B on 30/10/2016 11:00:32
Thank you for your input. Please forgive my impatience, but I am tempted to get my son to make a video of me trying to make posts on this broken phone. The screen constantly freezes up, or directs me to wrong page, and I'm uncertain about the future of my internet connection.OK, but just bear in mind that I almost didnt come back.
The question was concerning if decreasing the length of a standard second proportionally to increased energy as the Planck data curve diverges from the classical curve in the higher frequencies - or indeed adding to the length of a standard second proportionally to decreased energy where the Planck curve diverges from the classical curve in the lower frequencies - and if measuring joules per second added to the blackbody via these variable seconds as energy is added, would the Planck data be rendered linear?This is actually 2 separate questions:
The question is not concerning if doing so is appropriate, just if Planck's data could then be linear instead of quantised.
1. Could Plank curve be made to match the Rayleigh curve.
Just a slight digression, but it is relevant. The popular science press and others who should know better gloss over the history of this issue which goes back to the laws of thermodynamics, Kirchoff, Boltzman etc, so a lot has to change in order for Planks findings to change. Contrary to myth, Planck didn't overturn Rayleigh, the latter had put up the law as a 'look how ridiculous this is', he never believed it could be true.
So to the maths.
Curves are determined by the interaction of the components in the formula. Wherever you have a bump in the data you can guess at least 2 things are interacting whereas curves like Rayleigh can be derived from one.
Simple eg. Roll a dice, because all the numbers are equally likely you will get a distribution with no hump.
Now roll 2 dice and add them together, the most common number is 7 all the other numbers are less frequent – you have a hump in the data.
Now I'm not suggesting that there are probabilities here but just a simple explanation without using maths formulae that the interaction of 2 factors is what causes the hump, so changing frequency/time doesn't do it. To make a change you have to go back to thermodynamics, Kirchoff's work etc and start looking for the issues there. There is too much that is interdependent.
2.Would this linearise the data
The thing that is missing from E=hv in most quotes is n, more correctly E=nhv where n is an integer. Altering v doesn't change n, it is still there. Again you need to go well back to Planks work on oscillators and try to get rid of it, he tried and boy did he try hard!
Post by: jeffreyH on 30/10/2016 11:11:44
2.Would this linearise the data
The thing that is missing from E=hv in most quotes is n, more correctly E=nhv where n is an integer. Altering v doesn't change n, it is still there. Again you need to go well back to Planks work on oscillators and try to get rid of it, he tried and boy did he try hard!
Now I understand!
Post by: jeffreyH on 30/10/2016 11:25:20
https://msu.edu/~churchcl/quantum/quanta.html (https://msu.edu/~churchcl/quantum/quanta.html)
Post by: jeffreyH on 30/10/2016 11:51:52
What is interesting is if you take the diameter of a Planck mass black hole (4 Planck lengths) and divide this into 1 light second you can then use ratio to obtain a maximum frequency. Taking 4 Planck lengths as the Compton wavelength for the black hole. So with n =1 you get 1*h*v giving the energy that would be emitted by the black hole if this was possible. This can then be associated with Beckenstein entropy.
Post by: timey on 30/10/2016 15:10:01
What kind of logic would say that a cold clock won't tick, and are you seriously suggesting that you would think that I had thought this? (I suppose I should feel all rosy and cheery that you would infer that you ever did have warm feet about my theory)
Yes there are molecular frequencies and there are atomic frequencies to be thought about.
Colin was saying that time dilation/contraction can only happen when viewing another reference frame than your own. (please see NIST 2010 ground level cesium clock experiments where 2 clocks are running at different rates 1 metre apart). I said that time can be seen to be moving at different rates for molecular structures that are subject to heat or cold, ie: they decay faster or slower, and mentioned that temperature would affect a cesium atomic clocks frequency. Fact is that the molecular frequencies will indeed affect the atomic frequencies with their associated energies, but as to how this ties into my theory of time, to be truthful I haven't got a clue Evan. What I do know is that when a blackbody is exposed to temperature, radiation is emitted.
Jeff - could you please have an internal moment of reflection as to why you are becoming angry and uptight? It is clearly obvious that Planck's h constant is not fudged maths. Its highly relevant. However, Planck did try very hard to make his data fit a classical continuum, and in his attempts to do so, he split the energy input into sections to spread the data as to requirement, and the value of his h constant emerged. True or false?
Bored Chemist - thanks for joining the discussion. Yes time is a real phenomenon, and no, one cannot arbitrarily change its rate. However current physics has yet to possess a coherent theory of time. Currently the phenomenon of time is viewed by quantum and SR as having a universal present. Quantum is measured via the standard second, and infers that within perturbations of time, that there are alternative states that a system may be operating within, many worlds view, etc, and SR refers its time anomalies back to a universal present. But GR suggests that the present is only a locality.
So we can see that within the remit of currently held time dilation/contraction notions that a standard second may be considered as having an increased or decreased length of duration.
The argument most definitely exists that it may not be 'appropriate' to attempt to linearise Planck's data by lengthening the standard second where the Planck data curve diverges from the classical in the lower frequency region, or shorten the length of a standard second where the Planck data curve diverges from the classical in the higher frequencies - but would doing so, and then measuring the input energy via these variable seconds linearise Planck's data?
Colin - good, glad to see you back, I'm chewing over your post now.
Jeff - good, glad you understand! I'm chewing over your recent posts now too...
Post by: Bored chemist on 30/10/2016 15:15:34
Post by: jeffreyH on 30/10/2016 15:17:22
Post by: timey on 30/10/2016 16:08:12
If the addition of variable seconds to the interpretation did not iron out the quantised nature of Planck's data, it would without doubt be a pointless approach.
If the addition computes as a mathematical equivalent of current understanding, then it will be mathematically equivalent for different reasons than are currently held, and we can then take those reasons to the remit of Hubble's red shift velocities and reassess.
Jeff - your tone can be very contentious and accusatory, and this is the reason I find you to be perhaps angry with me and uptight. You have indeed taught me a lot over the last 2 years, more from reading your other posts than from your replies to me, where I feel that you constantly misunderstand my questioning of current status quo as being a lacking in my understanding of current status quo, and in that this is your perspective of me, you miss the implications of what I'm suggesting... For instance you cannot possibly correct my misconceptions concerning my model of a cyclic universe based on someone else's model of a cyclic universe.
Please understand Jeff, I am not stating that I have the ultimate theory of everything, everyone else is stupid. Blah, blah. All I'm saying is that I have a model that might be a mathematical viability in that it should remain the mathematical equivalent of GR, and quantum, but for different reasons.
Post by: alancalverd on 30/10/2016 16:23:52
Post by: timey on 30/10/2016 17:29:31
The benefits of ironing out the quantised nature of Planck's data are: that in keeping the distance of the wave'length' as per where the data lines do not diverge as a constant, one will always know the position of a photon particle along the path of this constant wave'length', because the per second part of the speed of light constant, is rendered variable. No need for perturbations of time to determine position.
Post by: Colin2B on 30/10/2016 18:43:34
Colin was saying that time dilation/contraction can only happen when viewing another reference frame than your own. (please see NIST 2010 ground level cesium clock experiments where 2 clocks are running at different rates 1 metre apart).
What I actually said was:
Although time varies between reference frames and places of differing gravity, there is no indication that it varies within an inertial frame.
The clocks are in differing gravity, strictly speaking different accelerating frames.
Post by: timey on 30/10/2016 19:24:57
And yes, your concern that the basis for lengthening or shortening seconds under the circumstances I suggest is valid with respect to the premise of relativity...
I am simply suggesting that in calculating probability of position in quantum, what I'm suggesting is exactly what the process of perturbating time is already adding. I'm just giving the process a physical reality. (potentially)
Post by: alancalverd on 30/10/2016 20:48:21
Post by: timey on 31/10/2016 01:18:24
So we have a situation where the increasing of temperature is causing frequency changes in the molecular structure of the blackbody via translational motion, and these motions cause electronic transitions in atoms, and photons are subsequently emitted.
Change the temperature input to the molecular structure and the frequency of the photons are changed accordingly...
Here we can see that the frequency changes in the photons are an indirect result of frequency changes in the molecular structure.
We can also see that the notion of a waves length is dimensionless. Although the length of the light wave is proportional to that frequency of lights energy, the length of a wave in terms of a distance is completely meaningless as light of any frequency travels the same distance in the same time period.
And... light of any frequency, ***after it has been emitted***, will not change its frequency unless exposed to changes in the gravitational field.
The sun is our closest natural light source. It is not moving away from us in the sense that other natural light sources are thought to be doing, as we are in orbit around it. The light from the sun is being red shifted away from the suns gravitational field, and then blue shifted into earths gravitational field. There is no significant Doppler effect here, (is there?), just gravitational shift. We know the distance to the sun and the speed the light travels at, the length of a wavelength remains dimensionless, and we still hypothetically know the photons position.
Taking the situation intergalactic, we now have Hubble's redshifts velocities to contend with. This has given the length of a wavelength a dimension. Wavelengths are now being so called stretched by velocity that light source is moving away from us at... And furthermore the distance the wavelength is travelling through is also expanding at speeds that can exceed the speed of light... Where is the photon now?
Post by: Colin2B on 31/10/2016 10:15:11
We can also see that the notion of a waves length is dimensionless. Although the length of the light wave is proportional to that frequency of lights energy, the length of a wave in terms of a distance is completely meaningless as light of any frequency travels the same distance in the same time period.Can you please explain how a wavelength is dimensionless and meaningless, I don't follow your reasoning.
You've obviously thought this through carefully, so could you also describe how, if wavelength is dimentionless and meaningless, you explain Chladni patterns, diffraction etc.
Post by: alancalverd on 31/10/2016 10:50:07
Post by: jeffreyH on 31/10/2016 11:17:57
Alas there are no quantum discontinuities in the spectrum of a black body, so there's something wrong with the argument here.
I am not sure what this relates to, can you elaborate.
Post by: alancalverd on 31/10/2016 12:20:07
Quote
these motions cause electronic transitions in atoms, and photons are subsequently emitted.
even the word "subsequently" is wrong!
Post by: timey on 31/10/2016 15:46:27
The reason why the cyclic count of a wavelength is dimensionless is because of the constancy of the speed of light. It does not matter what the cyclic count is, the light always travels the same distance at the same speed.
However, the cyclic count of wavelength is not entirely dimensionless. Apart from being proportional to energy, the rate of the count is being held relative to the duration of a standard second. As the speed of light is also being held relative to the duration of a standard second one would have thought we were all in the clear.
By adding velocities to the cyclic count of waves as Hubble did, the cyclic count of waves has been given a dimension of distance, and where light cannot, under the remit of the constancy of the speed of light, travel those 'distance' waves, it is then said that the fabric of space itself is stretching.
I'm at it a bit sketchy perhaps, but I can iron out any terminology if objection arises...?
P.S. I'd also like to know what you mean Alan, and:
"electronic transitions in atoms are accompanied by the emission of photons (radiation)".
Post by: alancalverd on 31/10/2016 16:12:23
You really do need to get a grip on dimensional analysis before wading into the waters of physics.
Post by: jeffreyH on 31/10/2016 16:21:15
Post by: timey on 31/10/2016 16:58:28
Jeff - yes, you were referring to frequency. So am I correct in my interpretation that the count of frequency is dimensionless?
Post by: Colin2B on 31/10/2016 17:53:10
Jeff - yes, you were referring to frequency. So am I correct in my interpretation that the count of frequency is dimensionless?The count is dimensionless (remember Sesame Street), frequency and wavelength are not.
I realise you think we're products of our misinformed education and totally unable to think outside the box (well, he keeps telling us so) but if any of your ideas ever come to being judged it is this lack of understanding of basic principles that will lead to you being mis-judged.
I only say this because I feel you and your ideas would benefit from spending some time looking at these basics, it's not just terminology, it helps to keep ideas clear.
Post by: timey on 31/10/2016 18:20:57
I never watched sesame Street, and indeed did not experience TV in the home, apart from a brief 4 months before travelling to India, until 1983.
I do not know why you keep referring to me in relation to Thebox. It's highly annoying. The dude can't even comprehend that there is an 8 light minute delay between the sun and the Earth. I don't want to be insulting Thebox or anything, I have respect not to cause upset, but really Colin?
Yes the count is dimensionless, but the duration of time the count is held relative to is not dimensionless. True or false?
Post by: Colin2B on 31/10/2016 23:01:06
I do not know why you keep referring to me in relation to Thebox.Not a reference to you at all, but aimed at the box - perhaps unfairly. I have a great deal of respect for the box, he does try to think, but gets bogged down by misunderstanding what he is observing. I'll try not to comment on him.
Yes the count is dimensionless, but the duration of time the count is held relative to is not dimensionless. True or false?Yes, that's what I said. The count of cycles is dimensionless, the frequency (cycles per unit time) is not.
What confused me was your suggestion that wavelength is dimensionless and meaningless. I can see now that you were not talking about wavelength.
PS My education was also nonstandard, long story, but I left school with no qualifications - unless you count a leaving certificate which said little more than you've been here and left.
Post by: alancalverd on 01/11/2016 00:44:25
Alan - yes indeed, but the energy input to radiation frequency ratio is quantised as a result of the blackbody experiment data.
Nothing happens as a result of experimental data. It's the other way round: data is the result of what happens. E=hf is not a statement of quantisation as f can take any value in a continuum.
The confusion arises because Planck's Law is derived from the concept of electromagnetic radiation in a hypothetical conducting cubical box: this allows us to count the number of photons at any wavelength that has nodes at the walls of the box, and hence the energy density as a function of frequency within the hypothetical box. However to get from there to the emitted spectrum from an actual object, you have to integrate over all possible boxes in the object, which gives you a continuous function with no forbidden transitions.
Post by: timey on 01/11/2016 05:37:03
The frequency of wavelength doesn't really take on a meaningful dimension until velocities are attributed. Am I correct?
Alan - I'm giving what you've said some in depth thought before replying, but it occurs that a sphere is the most efficient shape. Would a blackbody conducted in a sphere give different data?
Post by: Colin2B on 01/11/2016 09:25:59
OK Colin - Thanks. Though I too like Thebox and I have no wish to hurt his feelings.By the way, he does understand that light takes the 8mins, he is confused by an interesting variation of Zeno's paradox.
The frequency of wavelength doesn't really take on a meaningful dimension until velocities are attributed. Am I correct?I'm having difficulty deciphering your sentence because you are still misusing wavelength, I think? What do you mean by frequency of wavelength? Frequency or cycles?
Frequency is very meaningful without considering velocity. Similarly wavelength.
Would a blackbody conducted in a sphere give different data?No, the shape of the body has no effect on the radiation curve. You need to reread Alan's post which explains it all.
Post by: alancalverd on 01/11/2016 10:13:16
Post by: Colin2B on 01/11/2016 11:24:39
Think flute (clean sinusoid) compared with a rectangular organ pipe.I like Alan's eg.
If you want to use Chladni you might imagine photographing 1000s of plates at different frequencies, if you then superimposed these photos you would see sand continuously covering the whole area but thicker in some places (like a bump in the curve). Not a perfect illustration, but you get the idea.
Post by: jeffreyH on 01/11/2016 15:09:20
Post by: timey on 01/11/2016 15:39:54
Alan - From what I gleaned from diagrams, the blackbody looks cylindrical and rounded at the ends. (I was thrown by your cubic description, but interested in the resulting thought train.)
The light does not change frequency in the cavity after it has been emitted though, right?
It is as the transferal motion at the molecular level causing electronic motion is increased by the applied temperature, that the emitted photons have more energy, right?
Jeff - just saw your post... Interesting.
Post by: alancalverd on 01/11/2016 16:11:21
Frequency is the count of cycles per unit time. Dimension is T^-1
Wavelength is the distance between peaks. Dimension is L
Keep it simple and use the same language as everyone else, if you want to be taken seriously.
https://en.wikipedia.org/wiki/Black_body is good, as is the Wikipedia entry for "thermal radiation".
If I was making a practical source of blackbody radiation it would probably be cylindrical rather than spherical so I could heat it evenly, and just accept the fact that it might be slightly biassed in the very long wave region.
Not sure what "The light does not change frequency in the cavity after it has been emitted" means. How can it, once it has been emitted?
Re: Jeff's post. Doppler shift is not a relativistic effect.
Post by: timey on 01/11/2016 16:40:22
Yes - the photons do not change frequency after being emitted, so does this mean that the shape of the blackbody, and how those light waves travel around inside it, is irrelevant to the data curve?
Post by: alancalverd on 01/11/2016 17:37:50
As I said, the optimum black body would be an infinite sphere at an absolutely contant temperature, with an infinitesimal hole, but a cylinder with hemispherical ends is generally good enough over a small spectral range.
Post by: timey on 01/11/2016 18:10:57
And yes, that's right, because a sphere is the most efficient shape. But the change in the frequency of the light is occurring within the changes in the molecular and atomic structure of the blackbody not the cavity. Correct?
Post by: alancalverd on 01/11/2016 22:57:30
But Hubble adds the aspect of velocity to the scenario.Obviously, if the source is moving wirh respect to the observer, you will see both Doppler and relativistic changes in the received frequency.
Quote
And yes, that's right, because a sphere is the most efficient shape. But the change in the frequency of the light is occurring within the changes in the molecular and atomic structure of the blackbody not the cavity. Correct?What change? Blackbody radiation is a continuous spectrum. If you aise the temperature the peak and upper limit shift towards a higher energy and the total photon flux increases. Atomic structure is not changed by temperatures below several zillion degrees. There are no molecules in a metallic structure.
Post by: timey on 02/11/2016 00:23:07
If it were then the data would not be quantised. So where in the process is the data becoming quantised?
The resulting data of the experiment suggests that it is the input that is delivered in packets. But what if the input is a continuum and there is another process occurring? If the addition of a continuum of thermal energy to a molecular structure can cause the atoms to emit photons of escalating energy, where E=hf, hwill be the value of that other process.
Where the data curved diverges in higher frequency region, (or lower frequency region) by attributing lesser (or extra) length of seconds to the process proportionally as energy is added (or decreased), a bit like wavelength being inversely proportional to frequency, except it would be a variable second, the length of which being inversely proportional to frequency, and using these variable seconds to calculate as the applied energy is increased, the value of h will have been transferred and the energy input 'can' be a continuum...
Wavelength can be calculated as hc/E, or c/f. With E=hf, I can see that the logic might work, but it's the maths that are the defining factor. Does what I suggest actually do what I suggest it does mathematically?
Post by: Colin2B on 02/11/2016 08:41:03
... by attributing lesser (or extra) length of seconds to the process proportionally as energy is added (or decreased), a bit like wavelength being inversely proportional to frequency, except it would be a variable second, the length of which being inversely proportional to frequency, and using these variable seconds to calculate as the applied energy is increased, ...... I can see that the logic might work, but it's the maths that are the defining factor. Does what I suggest actually do what I suggest it does mathematically?You can get the same effect in many different ways
12+3=15
3x5=15
If I drive 100 miles at 50mph it will take 2hrs. If I do the same distance in 1.5hrs do I suggest that time has changed or my speed? Effect is the same.
You need to show just cause, not just an effect.
Post by: alancalverd on 02/11/2016 08:50:51
Baffled by "the data is quantised" when it isn't. The data shows a smooth curve with no discontinutities if there is a sufficient photon flux, but if you look at one photon a a time, each has a specific energy. Think of graded sand and half-inch ballast: each grain has a fixed size and there will be a maximum probability of finding grains in a particular size range (around half an inch diameter) but there is no rule about forbidden sizes. What Planck showed is that you can calculate the actual continuum shape by assuming that the underlying phenomenon is quantised.
Post by: timey on 02/11/2016 12:15:48
http://www.nature.com/news/2010/100923/full/news.2010.487.html
Alan - what I'm doing is suggesting a means of calculating the classical shape via a means that (potentially) eliminates the underlying quantised nature.
Post by: alancalverd on 02/11/2016 13:07:12
Post by: timey on 02/11/2016 13:52:31
http://www.sci-news.com/astronomy/science-universe-not-expanding-01940.html
...and also because the maths of using variable seconds as appropriate in quantum will eliminate the need for perturbation theory to know position, and (potentially) eliminate the use of the Lorentz transformations to describe Doppler.
Post by: alancalverd on 02/11/2016 15:40:37
Post by: timey on 02/11/2016 16:10:34
No collapsing going on!
And one could imagine that in more complex arrangements, that a jittering of such distribution of resonating frequencies could cause the atom to stabilise by emitting a photon.
Post by: alancalverd on 03/11/2016 14:15:09
The beauty of the Heisenberg/Planck/Schrodinger approach is that it predicts what actually happens without resort to magic.
Post by: timey on 03/11/2016 15:58:51
If there is a time dilation of open space then the actual force of gravity will have a value far less than the current gravitational constant. In fact it would be the magnetic moment of the electron that will be the force that causes an attraction, and the repulsion from the nucleus that repels, and the time dilation of open space causing the rate of acceleration inbound from point of inertia, or the rate of deceleration outbound from point of repulsion- this insuring that the electron will never fall into the nucleus. (but as you know Alan, this is all hypothetical supposition of how an alternative might give same results)
Yes of course, if the maths I'm suggesting correspond, they will correspond with what is predicted and what happens... So why bother looking at alternate maths? Because it might lead to a deeper understanding that can answer and give physical cause for the phenomenon of quantum states... That's why.
(Hmmm, magic? Quantum states, many worlds, or just a case of an undiscovered counter directional gravitational time dilation? I'd go for many worlds myself, but I always did like a fantastical fairy tail, and its soooo much more exotic, when can we visit?)
Post by: alancalverd on 03/11/2016 18:25:38
Not all nuclei have a magnetic moment.
"Many worlds" is a mathematical model. Quantum states is what actually happens because we can measure it.
Post by: timey on 03/11/2016 19:25:22
On the basis that I've obviously been led astray by the contradictory story that is portrayed in popular science, TV documentaries, and scientific press, I am now very annoyed at these misrepresentations that I've bought into and think I might have to sue.
Thanks for straightening me out Alan. Good day!
Post by: timey on 03/11/2016 20:02:01
Many apologies. I'm sooo embarrassed...
Post by: hamdani yusuf on 14/01/2024 07:12:56
The reason E = hv is that h is joule second and v is cycles divided by seconds. Since cycles have no dimension you can simply cancel out the unit of seconds in the numerator and denominator leaving the dimension of the answer as joules. Note that cancelling a unit has no effect on the values in the equation. This is what we use dimensional analysis for. I have explained this in words rather than just using maths since that is how you requested answers.The unit for h is actually Joule second per cycle. Reduced Planck's constant, called ħ (h bar) has a unit of Joule second per radian. They have the same dimension, but different in numerical value.
Using ħ can reduce the number of symbols used in equations of quantum mechanics, where we need to write down 2π if h were used instead.
Post by: alancalverd on 14/01/2024 13:17:25
The unit for h is actually Joule second per cycle.
Er, no.
Quote
The SI units are defined in such a way that, when the Planck constant is expressed in SI units, it has the exact value h = 6.62607015?10−34 J⋅Hz−1
Since 1 Hz = 1/second, the term Hz-1 has the dimension of time, so h is simply 6.62607015?10−34 joule.sec.
h/2π = ħ is still joule.sec but 2π turns up in so many calculations that it is simply a more compact way of writing equations.
Post by: hamdani yusuf on 15/01/2024 12:05:35
The unit for h is actually Joule second per cycle.
Er, no.QuoteThe SI units are defined in such a way that, when the Planck constant is expressed in SI units, it has the exact value h = 6.62607015?10−34 J⋅Hz−1
Since 1 Hz = 1/second, the term Hz-1 has the dimension of time, so h is simply 6.62607015?10−34 joule.sec.
h/2π = ħ is still joule.sec but 2π turns up in so many calculations that it is simply a more compact way of writing equations.
Quote
The hertz (symbol: Hz) is the unit of frequency in the International System of Units (SI), equivalent to one event (or cycle) per second.If a wheel is turning at 1/2π cycle per second, then it's turning at 1 radian/cycle. Because 1 cycle = 2π radian.
https://en.wikipedia.org/wiki/Hertz
Post by: alancalverd on 15/01/2024 14:34:55
Post by: hamdani yusuf on 15/01/2024 14:49:18
Photons are not rotating, so radians are not involved. How many radians in a square wave? Or a piano string?You seem to forget about Fourier transform.
Post by: alancalverd on 15/01/2024 16:37:41
Hammond organs and power stations use a rotating shaft to generate various frequencies.
Post by: hamdani yusuf on 15/01/2024 21:18:36
I never forget Fourier, but photons and pianos have never heard of him.Do you think photons have square waves?
Hammond organs and power stations use a rotating shaft to generate various frequencies.
Do you think radio waves also consist of photons?
Post by: hamdani yusuf on 16/01/2024 07:20:14
But imagining light as point-like particles only brings more difficulties than it helps. You would be hard-pressed to explain many of its properties, like frequency, wavelength, polarization, diffraction, interference. On the other hand, imagining light as a mechanical wave like sound, wave on strings, water surface or membrane has its own drawbacks. I've shown that in my experimental video about blocking mechanism using microwave. My conclusion for now is that light is a different kind of wave, until I can find evidence showing otherwise.
Post by: alancalverd on 16/01/2024 10:07:22
None of which changes the dimensions of h or ħ : joule.second. 2π is a dimensionless constant.
Post by: hamdani yusuf on 16/01/2024 13:12:51
None of which changes the dimensions of h or ħ : joule.second. 2π is a dimensionless constant.Radian is indeed a dimensionless unit.
When you have the light frequency in radian/second unit instead of Hertz, you can multiply it with ħ instead of h to get the energy.
https://en.wikipedia.org/wiki/Radian
Quote
The unit was formerly an SI supplementary unit and is currently a dimensionless SI derived unit
Post by: alancalverd on 16/01/2024 17:38:11
Post by: hamdani yusuf on 17/01/2024 10:30:29
radian/sec is not a frequency but a rate of change of angle or heading. A "standard rate 1 turn" in an airplane is 0.0524 radian/second.Perhaps you should stick to commonly used definition of any concepts, unless you have a strong reason not to do so.
Quote
The radian per second (symbol: rad⋅s−1 or rad/s) is the unit of angular velocity in the International System of Units (SI). The radian per second is also the SI unit of angular frequency (symbol ω, omega). The radian per second is defined as the angular frequency that results in the angular displacement increasing by one radian every second.
https://en.wikipedia.org/wiki/Radian_per_second
Post by: hamdani yusuf on 17/01/2024 10:33:19
h/2π = ħ is still joule.sec but 2π turns up in so many calculations that it is simply a more compact way of writing equations.What do you think is the unit of ħ?
What's its value?
Post by: alancalverd on 17/01/2024 12:03:57
h = 6.62607015?10−34 J⋅Hz−1, so ħ = 1.054571817...?10−34 J⋅s or thereabouts.
I can't apologise for the irrationality of π.
Post by: hamdani yusuf on 17/01/2024 14:43:33
Post by: Origin on 17/01/2024 15:22:25
An antenna is oscillating at 1 billion radian per second. What's the energy of its photon?Seriously? What do you think the energy of a photon is from an antenna oscillating at 57.3 billion degrees per second? Does the question even make sense to you?
Post by: hamdani yusuf on 18/01/2024 02:13:36
If it wasn't obvious to you yet, I referred to the electric field in the antenna.An antenna is oscillating at 1 billion radian per second. What's the energy of its photon?Seriously? What do you think the energy of a photon is from an antenna oscillating at 57.3 billion degrees per second? Does the question even make sense to you?
Post by: alancalverd on 18/01/2024 16:09:44
I may have time to do the calculation later, but the dog needs a walk!
Post by: Bored chemist on 18/01/2024 16:29:16
109 rad/sec is a rate of rotation,Not necessarily.
https://en.wikipedia.org/wiki/Angular_frequency
In which case the answer isn't hard to calculate.
But I don't see any point.
Certainly not any point that justifies the thread necromancy.
Post by: alancalverd on 18/01/2024 17:22:32
Quote
The unit hertz (Hz) is dimensionally equivalent, but by convention it is only used for frequency f, never for angular frequency ω. This convention is used to help avoid the confusion that arises when dealing with quantities such as frequency and angular quantities because the units of measure (such as cycle or radian) are considered to be one and hence may be omitted when expressing quantities in SI units.
So why make life difficult by ignoring a convention?
Anyway I get 10-7 eV
Post by: hamdani yusuf on 19/01/2024 04:30:46
The question doesn't make sense. 109 rad/sec is a rate of rotation, so presumably we are looking at a betatron, not an antenna. The electron energy, and hence the energy of any photons emitted, depends on the radius of the torus.Photonic interpretation for Planck's law states that hf is the energy of one photon. Thus radius of the
I may have time to do the calculation later, but the dog needs a walk!
torus or antenna doesn't affect photon energy, as long as the frequency can be kept the same.
Post by: hamdani yusuf on 19/01/2024 05:28:50
For those who are not familiar with electronics in radio communications, I can give a more visually accessible example. A bar magnet is rotated with axis perpendicular to the magnetic field inside the magnet. The angular speed is 3000 rpm (rotation per minute) , which is typical for industrial motors. The question is the same, what is the photon energy radiated by the rotating magnet?If it wasn't obvious to you yet, I referred to the electric field in the antenna.An antenna is oscillating at 1 billion radian per second. What's the energy of its photon?Seriously? What do you think the energy of a photon is from an antenna oscillating at 57.3 billion degrees per second? Does the question even make sense to you?
Post by: alancalverd on 19/01/2024 11:38:54
Photonic interpretation for Planck's law states that hf is the energy of one photon. Thus radius of theBut it does affect the energy of the electron that generates the photon. It all gets a bit complicated as it's quite easy to get an electron up to 0.9c in a betatron, at which point the relativistic corrections become very significant.
torus or antenna doesn't affect photon energy, as long as the frequency can be kept the same.
Post by: hamdani yusuf on 19/01/2024 14:52:04
It supposed to affect the number of photons radiated by the antenna.Photonic interpretation for Planck's law states that hf is the energy of one photon. Thus radius of theBut it does affect the energy of the electron that generates the photon. It all gets a bit complicated as it's quite easy to get an electron up to 0.9c in a betatron, at which point the relativistic corrections become very significant.
torus or antenna doesn't affect photon energy, as long as the frequency can be kept the same.
How does relativistic corrections affect the photon frequency, and its energy?
Post by: Bored chemist on 19/01/2024 15:02:30
The question is the same, what is the photon energy radiated by the rotating magnet?Small.
3000 RPM is 50Hz so the photon energy is 50 times Planck's constant.
That's high school maths. Why have you reopened a long-dead thread to ask about it?
Post by: Bored chemist on 19/01/2024 15:05:01
Earth calling Alan.Photonic interpretation for Planck's law states that hf is the energy of one photon. Thus radius of theBut it does affect the energy of the electron that generates the photon. It all gets a bit complicated as it's quite easy to get an electron up to 0.9c in a betatron, at which point the relativistic corrections become very significant.
torus or antenna doesn't affect photon energy, as long as the frequency can be kept the same.
He's not talking about a betatron.
On one hand, that's a pity because it's more interesting.
On the other hand, it may be just as well because I think he'd make a mess of it.
Post by: alancalverd on 19/01/2024 17:29:24
It supposed to affect the number of photons radiated by the antenna.Nonsense. It affects the energy of the photons, not the number. More electrons -> more photons, electrons oscillating faster -> higher photon frequency and E = hf
Quote
How does relativistic corrections affect the photon frequency, and its energy?You need to apply relativistic corrections to the electron velocity as you increase its kinetic energy. If energy E gives it a speed of 0.9c, 2E won't make it travel at 1.8c,
Post by: alancalverd on 19/01/2024 17:32:15
He's not talking about a betatron.
He's talking about the angular speed of an electron. What else would you call a device that makes electrons orbit at a constant 109radians per second?
Post by: Eternal Student on 19/01/2024 22:15:06
For the oscillating electron in the antennae:
I'm making 109 radians/sec ---> frequency of 159 MHz. That's the top end of the radio frequencies, maybe early microwave.
Energy of a photon ≈ 1.05 x 1025 Joules = 6.6 x 10-7 eV
and I think @alancalverd suggested 10-7 eV.
I think you can get electrons to oscillate in an ordinary metal antenna at about this rate. If you go much faster you can start getting reflection of the electrons from the crystal lattice and stuff happens. I'm not an engineer but I believe the antenna gets hot and you end up with a spread of frequencies released rather than a clear emission at one frequency.
@Bored chemist seems to have done the rotating magnet and I agree with that answer.
A slightly more interesting question for the rotating magnet is as follows:
In open space, this rotating magnet is emitting radiation and thus losing energy. Where is that energy coming from? If the rotating magnet reduces its (rotational) k.e. - where is the torque coming from that makes that happen?
Best Wishes.
Post by: alancalverd on 20/01/2024 10:20:09
Post by: alancalverd on 20/01/2024 10:23:46
frequency of 159 MHz. That's the top end of the radio frequencies, maybe early microwave.No big deal - it's between the VHF (civilian) and UHF (military) aircraft comms bands.
Post by: paul cotter on 20/01/2024 13:57:19
Post by: paul cotter on 20/01/2024 14:02:48
Post by: hamdani yusuf on 20/01/2024 14:38:49
Have your read my first post here carefully?The question is the same, what is the photon energy radiated by the rotating magnet?Small.
3000 RPM is 50Hz so the photon energy is 50 times Planck's constant.
That's high school maths. Why have you reopened a long-dead thread to ask about it?
I posted it because I think the statement in bold above needs clarification. Just because a unit has no dimension, it doesn't mean that it can just be ignored. You will get different value if you use different units, such as radian, degree, grad, brad, etc.The reason E = hv is that h is joule second and v is cycles divided by seconds. Since cycles have no dimension you can simply cancel out the unit of seconds in the numerator and denominator leaving the dimension of the answer as joules. Note that cancelling a unit has no effect on the values in the equation. This is what we use dimensional analysis for. I have explained this in words rather than just using maths since that is how you requested answers.The unit for h is actually Joule second per cycle. Reduced Planck's constant, called ħ (h bar) has a unit of Joule second per radian. They have the same dimension, but different in numerical value.
Using ħ can reduce the number of symbols used in equations of quantum mechanics, where we need to write down 2π if h were used instead.
https://en.wikipedia.org/wiki/Angle#Units
Post by: alancalverd on 20/01/2024 15:03:54
I posted it because I think the statement in bold above needs clarification. Just because a unit has no dimension, it doesn't mean that it can just be ignored. You will get different value if you use different units, such as radian, degree, grad, brad, etc.Jeffrey didn't cancel "cycles" but "seconds", because [E] = ML2T-2 and [ν]= T-1 so [h] = ML2T-1.
[X] means "dimensions of X "
Conventionally, the frequency of radiation is always stated in Hz, to avoid the confusion you have made for yourself.
Post by: Eternal Student on 20/01/2024 17:17:27
Is a rotating magnet really emitting energy in free space? It certainly induces a current in a conductor, ......Inducing current in a conductor is a separate issue. It creates e-m waves in the region of space around itself, these will propogate. There is no reason it would stop emitting e-m radiation in free space.
In astronomy, pulsars are thought to emit some radiation due to rotation of magnetic dipoles which is just called "magnetic dipole radiation". (They can also emit other energetic particles, the magnetic dipole radiation is just one component of what they emit).
See section 6.1.4 of this online article for more discussion and forumulae to describe the amount of power radiated as dipole radiation. https://www.cv.nrao.edu/~sransom/web/Ch6.html - that's part of the 'National Radio Astronomy Observatory' website and should be safe and reliable enough.
That article also assumes this emitted energy does come from the rotational kinetic energy of the neutron star (read "neutron star" as "the big magnet"):
Magnetic dipole radiation extracts rotational kinetic energy from the neutron star and causes the pulsar period to increase with time.
To paraphrase all of this: A rotating dipole in free space really does seem to emit e-m radiation, furthermore this loss of energy may cause the rotating magnet to slow down and stop spinning over time. By Newtonian mechanics, some torque would seem to operate on the rotating object.
So the original question is not without some practical or experimental relevance.
In open space, this rotating magnet is emitting radiation and thus losing energy. Where is that energy coming from? If the rotating magnet reduces its (rotational) k.e. - where is the torque coming from that makes that happen?It's not a question I can easily answer and I would like to get some opinions on it. There are a few solutions already existing in some Q&A websites like Quora but their quality.... is... variable.
It might be something better put into a separate thread rather than side-tracking this one too much.
Best Wishes.
Post by: Eternal Student on 20/01/2024 17:43:25
Thanks also for the information about how high up the e-m spectrum you can go with an AC signal in an antenna.
We don't seem to use AC signal generators and an antenna to generate microwaves (we seem to use magnetrons in Microwave ovens etc.). If I've understood what you ( @alancalverd and @paul cotter ) have said, then we could generate microwaves with an AC signal generator and an antenna if we wanted to and the emission remains fairly sharply peaked at the desired microwave frequency instead of being a broader spectrum.
The next question in the back of my mind would be, why don't we just use that in a microwave oven? I'll guess a magnetron offers some cost advantage.
Best Wishes.
Post by: paul cotter on 20/01/2024 21:21:39
Post by: alancalverd on 20/01/2024 22:11:54
By Newtonian mechanics, some torque would seem to operate on the rotating object.This simply does not make sense. Consider a magnet rotating in an infinite vacuum. Where does its angular momentum go? Newtonian mechanics says it is conserved!
Post by: Eternal Student on 21/01/2024 00:44:19
Consider a magnet rotating in an infinite vacuum. Where does its angular momentum go? Newtonian mechanics says it is conserved!That is indeed an interesting problem to resolve. Just to be clear, I didn't make up the original situation and the questions that follow from it.
Starting from the beginning, a rotating magnetic dipole (or electric dipole) should generate e-m radiation. That's probably the first thing to check. Assuming this does happen, as indeed it does seem reasonable to do, we are left with a set of problems to resolve.
If the rotating magnet doesn't slow down, it keeps radiating some energy at a constant rate for ever. Where is that coming from? Does the magnet lose some mass until eventually it would be all gone?
In partial answer to your ( @alancalverd ) question, or at least as something you should consider, we have the following:
Photons are considered to be spin 1 particles in the standard model. As such, under a treatment of the situation with Quantum Electro Dynamics (QED) or compatible form of QFT, they can carry an intrinsic angular momentum. So there is a precedent for us to imagine that light may carry angular momentum.
In Classical electrodynamics it's harder to identify an angular momentum in light but not impossible. I'm told that as early as 1909, Poynting suggested that circularly polarized light has an angular momentum volume density associated with it.
It is not too difficult to imagine that circularly polarised light (where the orientation of of the E and B fields is rotating) can carry momentum and it now seems common to associate this classical representation of what is happening in terms of E and B fields with the Spin Angular Momentum (SAM) of photons exhibited in quantum theories like QED.
Wikipedia has an entire page discussing the various ways in which light can carry angular momentum:
https://en.wikipedia.org/wiki/Angular_momentum_of_light which inlcudes SAM and OAM.
Spin Angular Momentum of light is also discussed here, https://en.wikipedia.org/wiki/Spin_angular_momentum_of_light
Anyway, what I'm saying is that the angular momentum lost by a rotating magnet may be found in the e-m radiation that was emitted by it.
Best Wishes.
P.S. I'm getting very worried about side-tracking this thread. A moderator should feel free to move it. @hamdani yusuf should feel free to ask us to stop.
Post by: paul cotter on 21/01/2024 10:06:00
Post by: alancalverd on 21/01/2024 16:36:41
Starting from the beginning, a rotating magnetic dipole (or electric dipole) should generate e-m radiation.I think not. You need to accelerate a charge to generate a photon. So it is entirely possible that a spinning neutron star could excite radiation from any gas cloud, plasma or passing ion, but a magnet in an infinite vacuum won't.
Post by: Eternal Student on 21/01/2024 19:26:38
You need to accelerate a charge to generate a photon.It is a way but do really think it's the only way to generate e-m radiation?
Atoms generate e-m radiation when an electron changes orbit, we don't model this with electrons moving in well defined circular orbits and having a brief period of acceleration (and indeed we can't because they would always be emitting radiation if we did). EM radiation is emitted for reasons that are not explained by a charge having been accelerated.
A positron and an electron can annihilate and produce a gamma ray (well, a pair of them) and no acceleration of a charge was involved.
Maxwells equations do a fair job of describing electromagnetic radiation. There are terms linking ∇ x E to the time rate of change of B. If the B field is changing at a place then the E field must also change at that place. If a magnetic dipole with lengh L between the poles is rotating, then you can be fairly certain the B field at points in space along its path of rotation will be changing with time. An oscillating B field should generate e-m waves appearing at some distance from it under the same conditions that an oscillating E field should.
I'm sure you ( @alancalverd ) have said all of these things to other people in the past.
I don't have a copy of Griffth's Introduction to Electrodynamics to hand at the moment but apparently the section around equation 9.53 fully derives the e-m radiation that must arise in the far field from an oscillating magnetic dipole. This is a standard textbook and as an engineer you probably have a copy on the shelf. A rotating magnetic dipole isn't covered there but can be considered as two oscillating dipoles, one along the x-axis and one on the y-axis, oscillating with the same frequency but π/2 out of phase. Sometimes this is called a crossed oscillating quadrupole. There are a few places on the internet that may provide a complete derivation for the e-m radition arising from a rotating magnetic dipole. LaTex isn't working so I'm not going try it here.
In all honesty, I think it's fairly well established that a rotating magnet in free space will produce e-m radiation. However, if you can find fault with this result then I'm only too pleased to hear about it. If a rotating magnet doesn't produce e-m radiation, that'll be great - it would make most the remaining problems that arise from it go away.
Best Wishes.
Post by: paul cotter on 21/01/2024 20:17:10
Post by: hamdani yusuf on 22/01/2024 08:46:22
He didn't cancel cycle, but ignored it as if it wasn't there. You can't say always if there are exceptions.I posted it because I think the statement in bold above needs clarification. Just because a unit has no dimension, it doesn't mean that it can just be ignored. You will get different value if you use different units, such as radian, degree, grad, brad, etc.Jeffrey didn't cancel "cycles" but "seconds", because [E] = ML2T-2 and [ν]= T-1 so [h] = ML2T-1.
[X] means "dimensions of X "
Conventionally, the frequency of radiation is always stated in Hz, to avoid the confusion you have made for yourself.
Why can't someone state frequency in other units, such as kHz, THz, rpm, etc?
Quote
Frequency (symbol f), most often measured in hertz (symbol: Hz), is the number of occurrences of a repeating event per unit of time.[1] It is also occasionally referred to as temporal frequency for clarity and to distinguish it from spatial frequency. Ordinary frequency is related to angular frequency (symbol ω, with SI unit radian per second) by a factor of 2π. The period (symbol T) is the interval of time between events, so the period is the reciprocal of the frequency: f = 1/T.
https://en.wikipedia.org/wiki/Frequency
Post by: Bored chemist on 22/01/2024 13:44:59
Necromancy?, BC. I always thought necromancy was the divination of future events by dissecting a dead creature and examining it's entrails. This method has not as yet been ascertained to be effective!And someone tried to divine the future from the entrails of a long-dead thread.
It's not clear why.
Post by: Bored chemist on 22/01/2024 13:51:50
You seem to have invented a requirement for an orbit.He's not talking about a betatron.
He's talking about the angular speed of an electron. What else would you call a device that makes electrons orbit at a constant 109radians per second?
Pushing electrons to and fro in an antenna is cyclical (Particularly if the antenna is resonant.)
Possible answers to your question would include
https://en.wikipedia.org/wiki/Halo_antenna
and
https://en.wikipedia.org/wiki/Cavity_magnetron
Post by: Bored chemist on 22/01/2024 14:18:08
I also am fairly sure a rotating magnet would radiate em. It would have to rotate at quite a high speed to generate easily detectable radiation. I have been trying for several hours to mentally model the maths involved but I have failed so far. We normally look at charge acceleration to produce em as it is the most convenient method but one has to remember that the magnetic field is simply the electric field as seen from a different frame of reference.I never could do the maths, but I don't care.
I can get a toy magnet and put it on the turntable of a record player.
I can put a compass near it.
And I can watch the compass needle move.
If I'm lucky it will even rotate in synchrony with the turntable.
The force acting on the needle is electromagnetic.
The carrier of the EM force is the photon.
So, in this case, I know that a rotating magnet emits EM radiation. That force is periodic and thus there must be a component of the EM radiation with the same period.So it must have photons with energies corresponding to the frequency of rotation of the record player.
Re. "but a magnet in an infinite vacuum won't. "
If the magnet falls over in a non-existent forest with no observers, does it matter?
I contend that it will still emit EM radiation.
Imagine that we somehow have a magnet in an infinite empty universe.
We set it spinning (We know it is spinning, because an ant who happens to be standing on it notices the centrifugal effect).
After a while, and at some distance from the magnet, we magically call a compass needle into being.
Does the needle have to "wait" for EM radiation from the magnet to reach it, or is that changing field already there?
I can't see how it would so I think the magnet must have been emitting EM radiation all along.
But... if there's EM radiation, then there are photons.
And, if there are photons, the universe isn't empty.
So the solution may be that you can't have a rotating magnet in an empty universe.
All seems a bit esoteric..
But let's ignore nearly the whole of the universe and consider some hydrogen atoms.
Some of them have the magnetic dipoles of the electron and the proton aligned parallel, and in others it's antiparallel.
And if one happens to flip from the first state to the second, it emits a photon of about 21 cm wavelength.
That photon crosses space and is picked up many years later by a detector here on earth.
But we have only been constructing such detectors for about 100 years.
So, for a source more than 100 light years away, the detector had not been built when the photon was emitted.
I think that's close enough to " we magically call a compass needle into being." for the analogy to work.
An electron- with a magnetic diploe moment- was flipped and sent out EM radiation. It did so in a universe in which the detector did not exist.
You may say that, without the proton of the hydrogen atom, the energy of the photon would be undefined. Which is a fair point
But, at that point, I think you need to be able to do Laplace transforms and, as I said, I can't do the maths.
Post by: paul cotter on 22/01/2024 14:59:03
Post by: Bored chemist on 22/01/2024 15:38:59
Post by: paul cotter on 22/01/2024 16:17:20
Post by: Eternal Student on 22/01/2024 18:43:59
..one has to remember that the magnetic field is simply the electric field as seen from a different frame of reference...Well said, I very nearly added that to the list of things @alancalverd has probably said to other people.
I never could do the maths, but I don't care.You've made a good argument without the Mathematics.
LaTex still isn't working on the forum. However, there's a good derivation for the e-m radiation that must arise in the far field due to an oscillating electric dipole here: It's 24 minutes long and maybe nicer than working through the mathematics entirely on your own. Sadly, I don't think this You Tuber went further and made a video for section 9.5 of Griffith's which should be an oscillating magnetic dipole - but it's a reasonable template of how you would try and do the maths. It's completely un-necessary to watch the video but there's at least two people in the world who might care about the maths.
I have looked up the derivation of the wave equation via the Laplacian of the E or H field in phasor form and it is completely symmetric: a time variation in either produces an em wave.Thank you.
- - - - - - - - -
I think quite a few of us (maybe 3 people and that's about half the forum) recognise that a rotating magnet must emit e-m radiation.
If the magnet falls over in a non-existent forest with no observers, does it matter?Hmm... I've often wondered, if I'm alone in the woods and my wife and daughters cannot hear me, am I still wrong?
A monopole would throw a spanner in the maths where divH is required to be =0.You've already given the smart answer. Maxwells equations will not permit a magnetic monopole, so we can't use them and expect to get a sensible answer. As far as I know, quantum theories that do predict magnetic monopoles will have them behave much like an electric charge.
Best Wishes.
Post by: alancalverd on 22/01/2024 19:26:24
The force acting on the needle is electromagnetic.It's certainly magnetic, but where is the E field?
Post by: alancalverd on 22/01/2024 19:28:30
Pushing electrons to and fro in an antenna is cyclical (Particularly if the antenna is resonant.)but doesn't involve any rotation.
Post by: paul cotter on 22/01/2024 20:08:29
Post by: Bored chemist on 22/01/2024 20:33:14
And had anyone said it should?Pushing electrons to and fro in an antenna is cyclical (Particularly if the antenna is resonant.)but doesn't involve any rotation.
Post by: Jaaanosik on 22/01/2024 20:37:01
What do you mean 'to accelerate a charge to generate a photon'?Starting from the beginning, a rotating magnetic dipole (or electric dipole) should generate e-m radiation.I think not. You need to accelerate a charge to generate a photon. So it is entirely possible that a spinning neutron star could excite radiation from any gas cloud, plasma or passing ion, but a magnet in an infinite vacuum won't.
How does it work?
Post by: alancalverd on 22/01/2024 22:16:49
Post by: Jaaanosik on 22/01/2024 23:00:35
So let's have a 1 tesla bar magnet spinning at 1000Hz in a vacuum. Can anyone please describe its electromagnetic emission spectrum? I just can't visualise it!I suspect the spectrum will be frame dependent.
It depends who is 'looking'.
Post by: Bored chemist on 23/01/2024 00:21:55
So let's have a 1 tesla bar magnet spinning at 1000Hz in a vacuum. Can anyone please describe its electromagnetic emission spectrum? I just can't visualise it!Can you visualise the emission if you had an electric dipole rotating at that rate?
(Say I have an insulating horizontal disk with two metal balls on the perimeter on either end of a diameter and one is positively charged and the other negatively.) I rotate the disk at 1000 Hz.
Failing that, what about a (horizontal) dipole antenna being fed with 1000 Hz at the centre?
From the side, the disk looks like the dipole.
There's a slight complexity from the movement towards and away from you.
But if you consider two dipoles at right angles fed with two signals (both at 1KHz) and pi/2 radians out of phase, the emission will look like the pair of charges on a turntable (or a rotating dipole).
I'm pretty sure that the emission pattern from a magnetic dipole would be the same (give or take a phase/ polarisation change)
Post by: Jaaanosik on 23/01/2024 00:24:23
...Apparently there are virtual photons that enable fields, wave propagation, potential gradients, ...
The carrier of the EM force is the photon.
So, in this case, I know that a rotating magnet emits EM radiation. That force is periodic and thus there must be a component of the EM radiation with the same period.So it must have photons with energies corresponding to the frequency of rotation of the record player.
Re. "but a magnet in an infinite vacuum won't. "
If the magnet falls over in a non-existent forest with no observers, does it matter?
I contend that it will still emit EM radiation.
Imagine that we somehow have a magnet in an infinite empty universe.
We set it spinning (We know it is spinning, because an ant who happens to be standing on it notices the centrifugal effect).
After a while, and at some distance from the magnet, we magically call a compass needle into being.
Does the needle have to "wait" for EM radiation from the magnet to reach it, or is that changing field already there?
I can't see how it would so I think the magnet must have been emitting EM radiation all along.
But... if there's EM radiation, then there are photons.
And, if there are photons, the universe isn't empty.
So the solution may be that you can't have a rotating magnet in an empty universe.
All seems a bit esoteric..
But let's ignore nearly the whole of the universe and consider some hydrogen atoms.
Some of them have the magnetic dipoles of the electron and the proton aligned parallel, and in others it's antiparallel.
And if one happens to flip from the first state to the second, it emits a photon of about 21 cm wavelength.
That photon crosses space and is picked up many years later by a detector here on earth.
But we have only been constructing such detectors for about 100 years.
So, for a source more than 100 light years away, the detector had not been built when the photon was emitted.
I think that's close enough to " we magically call a compass needle into being." for the analogy to work.
An electron- with a magnetic diploe moment- was flipped and sent out EM radiation. It did so in a universe in which the detector did not exist.
You may say that, without the proton of the hydrogen atom, the energy of the photon would be undefined. Which is a fair point
But, at that point, I think you need to be able to do Laplace transforms and, as I said, I can't do the maths.
... and there are photons, energy wave packets, energy quanta changes, ...
The hydrogen 21cm photon is the quanta change.
The hydrogen atom as a proton-electron system has a binding energy.
When the 21cm photon flies away then the electron is bound to the proton with stronger force.
The amount of energy that left the system means that exactly this much more energy is going to be required for electron to fly away from proton when compared to the electron state prior to the flip and prior to the 21cm emission.
The same logic why quantum number 1 requires more energy to break electron free than higher quantum number.
Photons are tricky. :D
Post by: Eternal Student on 23/01/2024 00:55:26
What do you mean 'to accelerate a charge to generate a photon'?I'm not alancalverd.... but one way you could do this is with a device called a cyclotron. Put a charged particle like a proton in to a cyclotron with some initial velocity (and remember to switch the magnetic field generator on). Then e-m radiation will be emitted.
How does it work?
So let's have a 1 tesla bar magnet spinning at 1000Hz in a vacuum. Can anyone please describe its electromagnetic emission spectrum? I just can't visualise it!I guess you couldn't find Griffith's introduction to electrodynamics then. I don't have a copy of it either, which is a bit shameful, I may get one for Christmas if I'm lucky.
Page 150 and 151 of this online reference will suffice:
https://www.damtp.cam.ac.uk/user/tong/em/el5.pdf
That describes the far-field with quite arbitrary motion allocated to the magentic dipole m(t). Very close to the magnetic dipole many of the approximations used during the derivation would be poor, so the near-field can be quite different.
Is LaTex working yet? I'm going to try it and post to see how it looks....

If that's not working we'll be down to words.
Late Editing: A subsequent post was written and then removed. It'll take an hour to cover stuff without too many formulae, check it all through and try to avoid having too many errors etc.
Best wishes but will be back shortly.
Post by: Jaaanosik on 23/01/2024 02:11:14
Quote from: Jaaanosik on Yesterday at 20:37:01Adding energy to a charged particle, acceleration in a straight line, does not generate a photon.QuoteWhat do you mean 'to accelerate a charge to generate a photon'?
How does it work?
I'm not alancalverd.... but one way you could do this is with a device called a cyclotron. Put a charged particle like a proton in to a cyclotron with some initial velocity (and remember to switch the magnetic field generator on). Then e-m radiation will be emitted.
Changing trajectory direction of previously accelerated charged particle is the causality of the photon emission.
https://en.wikipedia.org/wiki/Bremsstrahlung
This is exactly 'flip of the charged particle spin', similar to 21cm hydrogen line spin-flip.
The magnetic field is only the tool that is causing the change of charged particle trajectory direction.
The charged particle spin-flip itself is the cause of the photon emission.
The charged particle is a system, photon (quanta energy change) is leaving the charged particle meaning the charged particle is losing energy, 'breaking'.
Post by: Eternal Student on 23/01/2024 04:05:40
Adding energy to a charged particle, acceleration in a straight line, does not generate a photon.I'm fairly sure it would generate some photons.
The link that you provided gives a formula for the power radiated when acceleration is parallel to the velocity of the charged particle.
When any charged particle (such as an electron, a proton, or an ion) accelerates, energy is radiated in the form of electromagnetic waves.
- Taken from a slightly different Wikipedia page. https://en.wikipedia.org/wiki/Larmor_formula
The only minor issue that will arise is whether all of this oscillation in E and B fields would always be recognisable as "a photon", a discrete packet of energy. Classically, it's an oscillation in the E and B fields but it should be a continous emission rather than something that occurrs in bursts at discrete places and times.
Best Wishes.
Post by: paul cotter on 23/01/2024 09:46:37
Post by: Jaaanosik on 23/01/2024 12:33:41
Hi.Adding energy to a charged particle, acceleration in a straight line, does not generate a photon.I'm fairly sure it would generate some photons.
The link that you provided gives a formula for the power radiated when acceleration is parallel to the velocity of the charged particle.
When any charged particle (such as an electron, a proton, or an ion) accelerates, energy is radiated in the form of electromagnetic waves.
- Taken from a slightly different Wikipedia page. https://en.wikipedia.org/wiki/Larmor_formula
The only minor issue that will arise is whether all of this oscillation in E and B fields would always be recognisable as "a photon", a discrete packet of energy. Classically, it's an oscillation in the E and B fields but it should be a continous emission rather than something that occurrs in bursts at discrete places and times.
Best Wishes.
This comes back to 'who is looking'.
(https://i.imgur.com/nhZe4PZ.png)
What is a straight line acceleration in one inertial frame is a curved acceleration in other moving frames.
The rest frame of the plates, in the middle, an electron accelerates in a straight line from the middle of the blue plate, cathode, to the middle of the red plate, anode.
The K'1 frame in which the plates are moving to the left,
and K'2 frame in which the plates are moving to the right,
the end-result is curved trajectory accelerations in K'1, and K'2 frames.
Post by: hamdani yusuf on 23/01/2024 13:09:12
This comes back to 'who is looking'.Interesting.
Is a stationary charged particle release photons when seen by an inertially moving observer?
What about a stationary magnet?
Post by: Eternal Student on 23/01/2024 16:52:10
you said to use LaTex. I had no clue what you were talking about and I should have asked-It's a markup language especially intended for mathematical symbols.
You may already have noticed that if you want to underline something in this forum, a set of tags appears around what you were trying to underline:
Code: [Select]
To [u]underline[/u] you enclose the start and end tags [u] and [/u] around the thing you want to underline.
That displays like this:To underline you enclose the start and end tags and around the thing you want to underline.
You can see how the tags have been removed and would just become a long underline around the word "and".
You could also use tags that mark the start and end of a block of LaTeX code (The capital letters in the acromym LaTeX, are supposed to be as shown, some capitals here and there although I often write LaTEX).
Code: [Select]
the [tex] and [/tex] tags mark the start and end of the LaTeX code block.
[tex] \sum \limits_{i=1}^n {3i} [/tex]
Inside of the LaTeX code block you can use code that signifies or "marks up" the symbols you want.
The bit of code above would have produced a Sigma symbol for a sum over an index i from 1 to n of terms 3i.
It would have looked like this:
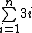
At the moment, whoever last edited, or updated the forum software may not have realised how LaTeX is supposed to work. I don't honestly know what happend. The buttons that appear along the top while you're creating a forum post all work.... so it just so happens you can press a button to get precisely what I've shown above.... You can get the sum from i =1 to n of terms 3i. However, if you change any of that, for example to display the sum from i =1 to 2n then it displays nothing.... well a numerical code linking to a gif image that the computer never generates.
Code: [Select]
This should be the sum from i=1 to 2n of terms 3i.
[tex] \sum \limits_{i=1}^{2n} {3i} [/tex]
This is what it produces:

If you can see what I mean... they may have thought.... "ahh... I have to get all the buttons to work"..... instead of "ahh.... I'm supposed to support the full use of LaTEX markup language".
In the old days we had full LaTeX support, now we have 8 buttons for just 8 things you can display.
Best Wishes.
Post by: paul cotter on 23/01/2024 18:07:49
Post by: Eternal Student on 23/01/2024 18:35:32
the end-result is curved trajectory accelerations in K'1, and K'2 frames.That is an interesting situation but I'm not sure what differences you are trying to imply would be seen.
Take some care to distinguish between a curved trajectory and an acceleration which is altered or "curved" as you phrased it.
The acceleration experienced by the charged particle is the same in all of those inertial reference frames.
The acceleration vector is a in frame K'1 and also the same vector in K'2 and the original frame which I'll call K0. For convenience let's say the acceleration is in in the z-axis direction, it will be in the z-axis direction for every frame and assuming the gap between plates and the potential across them remains constant, this acceleration is just a constant vector a independant of time for every experiment.
The acceleration is not curved or altered in any way.
Now we can imagine the experiment is run theree times, where the plates are at rest in the three different frames (and the charged particle is initially at rest on the blue plate) and you also maintain observers at rest in the frames K'1, k'2 and K0 for every experiment. Then the observers will each see two experiments where the charged particle takes a curved trajectory and only one experiment where it was straight.
The Larmor formula ( https://en.wikipedia.org/wiki/Larmor_formula ) depends only on the acceleration and not on whether the path was straight or curved. Using the simple Larmor formula which ignores relativistic effects (so the particle never reaches a high speed. Late Editing: and also we make no relativistic correction between frames, all relative speeds << c ) we see that every observer records exactly the same total amount of power radiated in the e-m field in every experiment.
There will be some differences in the e-m radiation. We have a Doppler effect, so if the apparatus and particle are moving toward an observer for half the experiment and then away for the other half, the radiation incident on them changes frequency and energy content. However, if they just collect all the radiation coming off the particle (for example construct a sphere around the apparatus that moves with the apparatus, and just measure the total power incident on that sphere at all times) the Larmor formula applies and everyone sees the same total radiated power.
Is a stationary charged particle release photons when seen by an inertially moving observer?No. If the charged particle is at rest in some inertial frame, then an observer at rest in any other inertial frame records the same acceleration for the charged particle (that will be 0 since it was at rest in some inertial frame). So the Larmor formula shows 0 e-m radiation from it.
If you wish to include General Relativity where you can examine non-inertial or accelerated reference frames properly then you could get some more interesting effects. There is the Unruh effect for example ( https://en.wikipedia.org/wiki/Unruh_effect ) together with associated Unruh radiation (which is a bit more controversial again). An accelerated particle, let's say a detector, may detect photons that were not there in an inertial frame.
Best Wishes.
Post by: Jaaanosik on 23/01/2024 18:58:34
Hi.Do you know that magnetic field generated by moving charged particle is velocity dependent?the end-result is curved trajectory accelerations in K'1, and K'2 frames.That is an interesting situation but I'm not sure what differences you are trying to imply would be seen.
Take some care to distinguish between a curved trajectory and an acceleration which is altered or "curved" as you phrased it.
The acceleration experienced by the charged particle is the same in all of those inertial reference frames.
The acceleration vector is a in frame K'1 and also the same vector in K'2 and the original frame which I'll call K0. For convenience let's say the acceleration is in in the z-axis direction, it will be in the z-axis direction for every frame and assuming the gap between plates and the potential across them remains constant, this acceleration is just a constant vector a independant of time for every experiment.
The acceleration is not curved or altered in any way.
Now we can imagine the experiment is run theree times, where the plates are at rest in the three different frames (and the charged particle is initially at rest on the blue plate) and you also maintain observers at rest in the frames K'1, k'2 and K0 for every experiment. Then the observers will each see two experiments where the charged particle takes a curved trajectory and only one experiment where it was straight.
The Larmor formula ( https://en.wikipedia.org/wiki/Larmor_formula ) depends only on the acceleration and not on whether the path was straight or curved. Using the simple Larmor formula which ignores relativistic effects (so the particle never reaches a high speed ) we see that every observer records exactly the same total amount of power radiated in the e-m field in every experiment.
There will be some differences in the e-m radiation. We have a Doppler effect, so if the apparatus and particle are moving toward an observer for half the experiment and then away for the other half, the radiation incident on them changes frequency and energy content. However, if they just collect all the radiation coming off the particle (for example construct a sphere around the apparatus that moves with the apparatus, and just measure the total power incident on that sphere at all times) the Larmor formula applies and everyone sees the same total radiated power.
...
Best Wishes.
The magnetic field generated by an accelerated charged particle is changing in magnitude and direction because it is velocity/trajectory dependent.
K0 does not predict this directional change but K'1 and K'2 do predict the change.
Post by: Jaaanosik on 23/01/2024 21:14:14
(https://i.imgur.com/1PjXz3e.png)
(https://i.imgur.com/ohSm5vG.png)
(https://i.imgur.com/ULkrUkX.png)
The B field forms an ellipsoid around the moving charged particle.
When the velocity grows the ellipsoid flattens and the B grows.
The growing B field causes spin like effect around the charged particle.
When the velocity changes direction the ellipsoid changes direction.
The moving frames predict a 'spin-flip' due to the 'curved' trajectory of the acceleration but the straight line acceleration frame does not.
There is a 4-tourque evolution disagreement between the inertial frames.
This is all textbook... somehow missed in our understanding of physics. ;)
Quote
That is an interesting situation but I'm not sure what differences you are trying to imply would be seen.This is a huge difference...
Post by: Eternal Student on 24/01/2024 00:43:43
Do you know that magnetic field generated by moving charged particle is velocity dependent?Yes (but thanks for the information).
The magnetic field generated by an accelerated charged particle is changing in magnitude and direction because it is velocity/trajectory dependent.
K0 does not predict this directional change but K'1 and K'2 do predict the change.
Yes, the magnetic and electric fields that the observers see will be different. How or why does that prevent a linearly accelerated charged particle from emitting radiation that you may detect as a photon of some frequency?
1. The base line of the B field at position x and time t0 does not prevent an oscillation above and below that being obtained as time evolves. We can still identify oscillations in the E and B fields which may be asscoiated with photons (conventional oscillatons of the E and B field at right angles and propogating away at c). Any oscillation in the fields can be decomposed into a sum of conventional sinusoidal oscillations of different frequencies.
For each observer, the frequencies and corresponding amplitudes involved in this Fourier decomposition of the oscillations at any given event (place and time allocated by observer according to their rest frame) could be different, that is not in dispute. As already mentioned there is a Doppler effect and we would fully expect the frequencies of radiation that an observer would see being thrown out in front of a moving charge will be different to the frequencies thrown out to the back of the moving particle.
2. Although the E and B fields may have different values for each observer, the transfer of some value from the E field to the B field changes the power being transferred in the e-m fields in a complicated way, we have to examine the product of magnitudes |E| |B| and the orientation of the fields.
The total power radiated in the e-m field is obtained by examining the Poynting vector S ~ E x B.
3. The Poynting vector can still be different at various events (an event = place and time) for observers at rest in different frames, that is also not in dispute. However, the integral of the Poynting vector over a spherical surface around the apparatus remains constant for all observers who are at rest in any one of the frames.
This is the information being encapsulated by the Larmor formula. It's not attempting to describe anything about the intensity or frequency of some photons that may be produced and emitted in various places or directions, only to determine the total radiated power carried in the E and B fields.
Just to be clear, there are no "photons" to be found in the classical theory of elctrodynamics, nothing is discretised like this. You can attempt to identify a stream of photons with a classical sinusoidal e-m wave of a particular frequency. Then the frequency of each photon is assumed to be the frequency of the e-m wave and the number intensity of photons (number of photons impacting on a surface per second) is considered to be proportional to the intensity (the square of the amplitude) of the classical e-m wave. That identification is what is usualy done.
For all observers, classical electrodynamics suggests the same total power is radiated. You need some quantum theory to identify any photons that may be emitted.
(Although I had started to discuss the conventional way to link classical electrodynamics to a model involving photons, it's not essential and the post is long, so it'll go under a spoiler).
Spoiler: show
The magnetic and electric fields for each observer will be different and that is not a problem. It doesn't prevent photons from being detected in some frame (e.g. the one where the particle was linearly accelerated and took a straight path). It merely changes the prevalence (frequency of detection) of photons of a given colour (oscillation frequency) that can be detected in various directions.
I hope that makes some sense.
Best Wishes.
Post by: hamdani yusuf on 24/01/2024 01:57:20
The only minor issue that will arise is whether all of this oscillation in E and B fields would always be recognisable as "a photon", a discrete packet of energy. Classically, it's an oscillation in the E and B fields but it should be a continous emission rather than something that occurrs in bursts at discrete places and times.Someone may argue that the issue is fundamental rather than minor.
Post by: hamdani yusuf on 24/01/2024 03:55:49
The unit for h is actually Joule second per cycle.
Er, no.QuoteThe SI units are defined in such a way that, when the Planck constant is expressed in SI units, it has the exact value h = 6.62607015?10−34 J⋅Hz−1
Since 1 Hz = 1/second, the term Hz-1 has the dimension of time, so h is simply 6.62607015?10−34 joule.sec.
h/2π = ħ is still joule.sec but 2π turns up in so many calculations that it is simply a more compact way of writing equations.
What do you think about this Wikipedia entry?
Quote
In many applications, the Planck constant
ℎ naturally appears in combination with 2π as ℎ/(2π), which can be traced to the fact that in these applications it is natural to use the angular frequency (in radians per second) rather than plain frequency (in cycles per second or hertz). For this reason, it is often useful to absorb that factor of 2π into the Planck constant by introducing the reduced Planck constant[38][39]: 482 (or reduced Planck's constant[40]: 5 [41]: 788 ), equal to the Planck constant divided by
2π[38] and denoted by ħ (pronounced h-bar[42]: 336 ).
https://en.wikipedia.org/wiki/Planck_constant#Reduced_Planck_constant_%E2%84%8F
Post by: hamdani yusuf on 24/01/2024 04:12:56
QuoteQuote from: hamdani yusuf on Yesterday at 13:09:12No. If the charged particle is at rest in some inertial frame, then an observer at rest in any other inertial frame records the same acceleration for the charged particle (that will be 0 since it was at rest in some inertial frame). So the Larmor formula shows 0 e-m radiation from it.
Is a stationary charged particle release photons when seen by an inertially moving observer?
Let a positively charged particle staying still at Cartesian coordinate (0,1).
An observer moving along x axis from left to the right at 1 m/s.
We can think of the implications of this situation:
Before passing the point of origin, he feels increasing electric field.
After passing the point of origin, he feels decreasing electric field.
Changing of electric field is often said to create magnetic field.
The rate of change of the electric field in this case is not constant.
Which means the magnetic field is also changing.
Changing of electromagnetic field is often called radiation.
Among the statements above, which one(s) do you think is wrong?
Post by: alancalverd on 24/01/2024 08:30:08
What do you think about this Wikipedia entry?
Here's what Wikipedia thinks about it (panel at the top right of the article)
Reduced Planck constant
Common symbols
ℏ
SI unit joule-seconds
exactly the same units as h
Post by: hamdani yusuf on 24/01/2024 09:07:48
Not exactly. See the panel on top of what you quoted.What do you think about this Wikipedia entry?
Here's what Wikipedia thinks about it (panel at the top right of the article)
Reduced Planck constant
Common symbols
ℏ
SI unit joule-seconds
exactly the same units as h
Planck constant
Common symbols
ℎ
SI unit joule per hertz (joule seconds)
Post by: alancalverd on 24/01/2024 10:16:38
Post by: hamdani yusuf on 24/01/2024 12:24:41
Post by: Bored chemist on 24/01/2024 12:50:02
Does the unit of the radiation frequency have no effect on the numeric value of their quantum of energy?The number of joules of energy which a photon carries doesn't know or care if you measure frequency in 1/seconds or 1/weeks
The value of Planck's constant (h) would change.
Post by: alancalverd on 24/01/2024 16:34:24
Does the unit of the radiation frequency have no effect on the numeric value of their quantum of energy?No. The unit of energy is joules or electron volts and the number is (by definition) fixed for any quantum.
Post by: alancalverd on 24/01/2024 16:37:58
doesn't know or care if you measure frequency in 1/seconds or 1/weeksSchlumberger used to boast that they could supply customised oscilloscopes "calibrated in millifurlongs per microfortnight", if you wanted.
Post by: Eternal Student on 24/01/2024 16:43:47
I'm sorry if this isn't what you ( @hamdani yusuf ) wanted. It takes hours to write replies like this, so I've got to try and keep it short even if there's a risk of sounding curt.
Among the statements above, which one(s) do you think is wrong?The following one:
Changing of electromagnetic field is often called radiation.
No it isn't. At best it's an indication there may be some propagating radiation. If you can identify a propagating wave in the E and B field with appropriate properties (sinusoidal, E orthogonal to B etc.) then you're on to something, you may have some light and hence some photons there. A photon can be called a form of radiation (along with alpha emission and other things).
More generally, radiation through the e-m field means there is some energy being radiated through the electric and magnetic fields. Radiated just means travelling away from the particle.
Quantum models suggest that photons are the only way you're going to get energy being carried away. So if you can find some radiation of energy through the e-m field then you can be fairly sure there will be some photons to be found travelling away from the particle. The converse doesn't have to hold. If there's no radiation of energy from the particle then (i) there may not be any photons BUT it could also be (ii) that there are some phtons, an equal number travelling in and being absorbed as those being thrown out (for example).
Power is radiated through the Electric and Magnetic fields if and only if The Poynting vector illustrates a net flow of energy out of an enclosed surface you can draw around the object.
It's apparent that in the frame where your positive charge was at rest, nothing too interesing happens.
In the frame where the observer was at rest, the particle will be moving left at 1m/s. Take a simple enclosed surface around the particle, a flat sided box around the particle will do nicely.
Draw the diagram of the E and B fields that exist around the particle. An earlier post from @Jaaanosik has made that easy for you where some pages from Griffith's appear.
You'll have a diagram a bit like this:
⊕ ⊕ ⊕
↑
◊
↓
? ? ?
where ◊ is the particle, Blue arrows ↑ ↓ are the electric field, red ⊕ and ? are the magnetic field going into or out of the page respectively. (The ? were dots but the forum won't support that character).
Determine the Poynting vector which is (proportional to) E x B as usual with your preferred rule (I use a right hand with fingers). Put that on your diagram.
You should end up with something a bit like this:
← ←
◊
← ←
Draw the field lines carefully and you'll have a slight downward slope on the top left arrow, slight upward slope on the top left arrow etc. They'll be symmetric in their up and down slope-ness.
Now put the box around it. The flux on the left wall is outward, the flux on the right wall is inward. These match in magnitude. There's no flux over the top and bottom or the "near to you" and "far from you" wall. Overall, there is no net flux of the energy into or out of the box.
Use a small box and you can see there's no net energy lost by the particle. No energy is radiated by the particle through the Electric and magnetic fields. This is in total agreement with the Larmor formula discussed earlier, there was no acceleration of the particle.
The diagram with the Poynting vectors may look like something is being sent out in one direction and coming in from the other direction but it's just a flow of energy in the E-M field at these points in space and is not always a propagating wave in the E and B fields that you could identify as some light. The situation is similar to having a magnet just sit still with the North pole pointing to a static positively charged particle. There will be some regions of space where E and B fields cross at some oblique angle and you have a non-zero Poynting vector, so some energy is flowing at that point in space. However, travelling e-m waves are not found at that point in space, we get no flashes of light from a static magnet pointing to a static charge. Take a moment to think about this, we can have a flow of energy indicated by the Poynting vector but there is no light to be found. In a D.C. electrical circuit, we can identify a flow of energy out of the battery and into the load it is connected to, there's definitely a flow of energy but we don't have rays of light (any frequency of it) shining out of the battery and being sucked up by the load. A flow of energy in the e-m field at a point in space can be quiet and invisble rather than forcing the production of photons.
We can go a bit further, if you wish. Since there's no net energy transfer in your example we don't expect to identify any photons being thrown out and propagating away from the particle. However, we can't formally exclude the possibility that there may be some, we may be in the situation where there are also some incoming photons.
So there may be some photons, you'd have to examine the E and B fields around the particle carefully to get more information. You're looking for the usual sinusoidal propagating waves as previously mentioned. You're free to do that if you wish. If you found any, then there would be an interesting situation. For example, light may be thrown out by the particle to the front in the direction of its travel, but some light must also appear behind it. The light behind it is interesting because it won't travel away, it would appear in space after the particle has passed and then chase after the particle and be absorbed. If you find a solution for the E and B fields that exhibit that behaviour, that'll be great and probably a publishable result.
Best Wishes.
Post by: hamdani yusuf on 25/01/2024 02:19:54
The value of h is 6.62607015x10−34Does the unit of the radiation frequency have no effect on the numeric value of their quantum of energy?No. The unit of energy is joules or electron volts and the number is (by definition) fixed for any quantum.
energy of a single photon is h.f.
If the photon frequency is 1 Hz, then its energy is 6.62607015x10−34 Joule.
If the photon frequency is 1 GHz, then its energy is not 6.62607015x10−34 Joule.
If the photon frequency is 1 rpm, then its energy is not 6.62607015x10−34 Joule.
If the photon frequency is 1 rad/s, then its energy is not 6.62607015x10−34 Joule.
Post by: Bored chemist on 25/01/2024 11:04:40
becauseThe value of h is 6.62607015x10−34Does the unit of the radiation frequency have no effect on the numeric value of their quantum of energy?No. The unit of energy is joules or electron volts and the number is (by definition) fixed for any quantum.
energy of a single photon is h.f.
If the photon frequency is 1 Hz, then its energy is 6.62607015x10−34 Joule.
If the photon frequency is 1 GHz, then its energy is not 6.62607015x10−34 Joule.
If the photon frequency is 1 rpm, then its energy is not 6.62607015x10−34 Joule.
If the photon frequency is 1 rad/s, then its energy is not 6.62607015x10−34 Joule.
The value of Planck's constant (h) would change.
Post by: hamdani yusuf on 25/01/2024 21:25:09
The familiar numerical value of h is in the unit of Joule/Hz, or Joule second/cycle. In different unit, the value would be different.
Post by: Bored chemist on 25/01/2024 23:02:49
Correct.And a grand total of zero people said otherwise.
The familiar numerical value of h is in the unit of Joule/Hz, or Joule second/cycle. In different unit, the value would be different.
Post by: hamdani yusuf on 25/01/2024 23:58:43
Are you sure?Correct.And a grand total of zero people said otherwise.
The familiar numerical value of h is in the unit of Joule/Hz, or Joule second/cycle. In different unit, the value would be different.
The only reason I resurrected this thread was because I saw someone said that the unit cycle in Planck's constant can be ignored. Subsequent discussion shows that he's not the only one.
Post by: alancalverd on 26/01/2024 18:10:22
he value of h is 6.62607015x10−34No, it is 6.62607015x10−34 joule.second. The number would be quite different if it was expressed in BThU.hours
Quote
energy of a single photon is h.f.Hardly a stunning observation, but true nonetheless.
If the photon frequency is 1 Hz, then its energy is 6.62607015x10−34 Joule.
If the photon frequency is 1 GHz, then its energy is not 6.62607015x10−34 Joule.
Quote
If the photon frequency is 1 rpm, then its energy is not 6.62607015x10−34 Joule.1 rpm or 1 rad/second is not a frequency, but a speed of rotation.
If the photon frequency is 1 rad/s, then its energy is not 6.62607015x10−34 Joule.
I haven't seen any rotating photons.
Post by: hamdani yusuf on 27/01/2024 02:22:45
No, it is 6.62607015x10−34 joule.second. The number would be quite different if it was expressed in BThU.hoursJoule second per cycle.
If the frequency is presented in kilocycle per second, the numerical value should be adjusted by 3 order of magnitude.
Post by: hamdani yusuf on 27/01/2024 05:16:40
1 rpm or 1 rad/second is not a frequency, but a speed of rotation.They are angular frequency, but frequency nonetheless.
I haven't seen any rotating photons.Haven't you learned something about circular polarization?
Even in linearly polarized light, the propagating electromagnetic wave forms a sinusoidal shape. This trigonometric function is often expressed in radian basis.
Post by: alancalverd on 27/01/2024 10:14:01
Quotefrom: alancalverd on Yesterday at 18:10:22Joule second per cycle.
No, it is 6.62607015x10−34 joule.second. The number would be quite different if it was expressed in BThU.hours
I give up.
Clearly the International Bureau of Weights and Measures (and everyone else) have no idea what they are talking about, our concept of dimensions, and the entire SI system of units, is faulty.
I suggest that, for the benefit of all mankind, you should dispel their ignorance with a direct communication to BIPM, Sevres, France (Quantum Mechanics Division).
Post by: hamdani yusuf on 27/01/2024 11:12:11
I give up.Your frustration is caused by your misunderstanding of frequency. Don't blame your own mistake to someone else.
Clearly the International Bureau of Weights and Measures (and everyone else) have no idea what they are talking about, our concept of dimensions, and the entire SI system of units, is faulty.
I suggest that, for the benefit of all mankind, you should dispel their ignorance with a direct communication to BIPM, Sevres, France (Quantum Mechanics Division).
Post by: Bored chemist on 27/01/2024 15:04:59
1 rpm or 1 rad/second is not a frequency, but a speed of rotation.Or it's a rate of change of phase angle.
In which case hamdani yusuf's statement makes sense.
Post by: alancalverd on 27/01/2024 15:54:21
Post by: Bored chemist on 27/01/2024 22:01:21
Then why does the SI unit not mention it?Feel free to ask them.
Post by: hamdani yusuf on 28/01/2024 10:34:31
Then why does the SI unit not mention it?SI organization consists of people, who view the world based on their contemporary science community. They are not infallible, and they have publicly changed the standards. They may have their blind spots, just like us human beings. That's why it's important to let different ideas to compete and be discussed to reveal any residual mistakes.
Post by: hamdani yusuf on 28/01/2024 11:15:41
1 rpm or 1 rad/second is not a frequency, but a speed of rotation.1 kilocycle per second, 1 Gigacycle per second, are also frequency. They also have 1/second dimension.
I haven't seen any rotating photons.
If you multiply them with Planck's constant, you get the number in kiloJoule and GigaJoule, respectively.
Those who are not familiar with communication science and engineering may not be familiar with negative frequency. If Fourier transform is applied to a linearly polarized monochromatic light, the result is two lines symmetrical to the y axis. One is positive and the other is negative with the same magnitude and distance from the point of origin. When the magnitude is different, we get elliptically polarized light. When the negative part is absent, we get a circularly polarized light.
Post by: Bored chemist on 28/01/2024 12:25:57
I haven't seen any rotating photons.Now I come to think of it, all the photons I have heard of carry angular momentum.