Post by: Stevie Bain on 26/09/2017 10:13:49
Can you make concentrated hydrochloric acid by mixing calcium chloride with water and boiling it down?
Can you help?
Post by: chiralSPO on 26/09/2017 13:19:24
1) CaCl2 adds Cl– to the solution, but doesn't appreciably change the concentration of H+ ions. Ca(OH)2 is slightly soluble in water, so one would expect concentrated solutions of CaCl2 to be mildly acidic, but Ca(OH)2 is also a strong base, and will react rapidly with acids like HCl. (If you are familiar with concepts like equilibrium and pKa, those explain very nicely how CaCl2 interacts with water)
2) Hydrochloric acid has a lower boiling point than water, so if you tried to boil it down to concentrate it, you would lose any of the HCl before most of the water started coming off. You might be able to get some HCl by distilling a concentrated solution of CaCl2, but I doubt it would be very concentrated.
That said, calcium chloride will react with sulfuric acid to produce hydrochloric acid and calcium sulfate, which will precipitate from solution:
CaCl2(aq) + H2SO4(aq) → 2 HCl(aq) + CaSO4(s)
Post by: chiralSPO on 26/09/2017 17:43:14
It is possible to produce HCl by distillation of a concentrated solution of FeCl3
in solution the following equilibrium lies far to the left:
FeCl3(aq) + 3 H2O(aq)
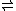 Fe(OH)3(s) + 3 HCl(aq)
Fe(OH)3(s) + 3 HCl(aq)but once the temperature is high enough, gaseous HCl leaves the system, driving the reaction to the right.
FeCl3(aq) + 3 H2O(aq) → Fe(OH)3(s) + 3 HCl(g)
But, I don't recommend trying to distill acid without the proper equipment, protective gear, and ventilation!
Post by: Bored chemist on 26/09/2017 17:48:45
It's a lousy way to do it, but you can.
If you dissolve CaCl2 in water you get a solution with chloride ions and it will contain hydrogen ions from two sources- firstly from the ionisation of water and secondly because the hydrated calcium ion [Ca(H2O)6] ++ is weakly acidic.
To a small degree, those hydrogen ions and chloride ions will "stick together" and form HCl.
So, if you distil that solution, some of the HCl will distil out.
However I doubt the effect is worth looking at.
Post by: chiralSPO on 02/10/2017 17:25:53
I burned myself with freshly distilled acid nearly a decade ago (it was anhydrous trifluoroacetic acid, so slightly nastier than concentrated hydrochloric acid, and about as bad as concentrated sulfuric acid). A single drop of acid that had stuck to the distillation head dripped out as I was disassembling the still, and landed right between my labcoat and glove (I had used extra-long heavy butyl gloves over my little nitrile gloves while handling the bottles of acid, but had foolishly just removed them to clean up after I thought I had transferred all the acid).
Anyway, I still have some significant scarring, and I was already right next to a sink with running water when it happened, so contact time was less than 2 seconds. See below (US 10 cent coin included for scale):
[ Invalid Attachment ]