Post by: krool1969 on 22/04/2013 03:31:22
Helium is a light, highly inert gas. If it's not manufactured where do our current supplies come from. Could it be made in large enough quantifies if we can ever develop fusion nuclear power?
Post by: damocles on 22/04/2013 04:47:14
Post by: CliffordK on 22/04/2013 08:37:50
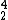 He) would be made in all nuclear power plants, but at the moment, the capture of this helium isn't economically viable.
He) would be made in all nuclear power plants, but at the moment, the capture of this helium isn't economically viable.There are several estimates that there is a looming helium crisis in 25 to 30 years as some of the current high concentration wells, and reserves run dry.
The helium is often recovered during the production of liquefied natural gas, but not all natural gas is liquefied before distribution.
Personally, I think that it is potentially valuable enough of a resource that it should not be sold as party supplies, although preserving it might mean slowing down both natural gas and helium production from some of the most prolific wells, as well as improving recovery from essentially all other wells.
Helium-3 (
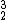 He), is far more rare on Earth, but may be an important nuclear fusion fuel in the future. It is produced in the sun, or as tritium decay product.
He), is far more rare on Earth, but may be an important nuclear fusion fuel in the future. It is produced in the sun, or as tritium decay product.
Post by: cheryl j on 23/04/2013 21:44:14
Post by: CliffordK on 23/04/2013 22:55:13
So is there anything else you can put in balloons? I for one, miss them.
Probably nothing that is not flammable, that would be less dense than air at 1 ATM. Could you make a mini hot air balloon?
Possibly Neon, but at half the density of air, it may not have enough lift for most ordinary balloons.
Hydrogen, of course, is lighter than air, and we have essentially an infinite supply of it. How dangerous is the explosion of say 1 gallon (4 liters) of hydrogen at 1 ATM? No doubt kids would quickly learn they could light them on fire.
Post by: damocles on 24/04/2013 01:05:09
So is there anything else you can put in balloons? I for one, miss them.
Comprehensive list of all lighter-than-air gases:
gas Buoyancy (relative to He = 100%) Drawbacks
hydrogen 108% explosively flammable
helium 100% slowly leaks from container
methane 52% explosively flammable
ammonia 48% highly toxic, corrosive, high affinity for water.
neon 36% prohibitively expensive
acetylene 12% flammable
6 others <5% various, but mainly not enough buoyancy.
Post by: cheryl j on 30/04/2013 04:06:24
Post by: CliffordK on 30/04/2013 08:11:00
Post by: syhprum on 05/05/2013 10:11:14
I notice that Argon is cheaper than Helium is it any good for lifting ?
Post by: damocles on 05/05/2013 10:35:13
How does Nitrogen rate for buoyancy ? I understand that the gas sold to inflate balloons is normally diluted the Nitrogen to reduce the cost while maintaining adequate buoyancy.
I notice that Argon is cheaper than Helium is it any good for lifting ?
Nitrogen is one of the gases that is only marginally lighter than air. The others are diborane, hydrogen cyanide, ethylene, and carbon monoxide.
A nitrogen balloon is quite impractical. Argon is 35% heavier than air, so again quite impractical.
The list I posted earlier in this thread is quite exhaustive.
Post by: syhprum on 05/05/2013 12:27:24
Post by: damocles on 05/05/2013 13:01:25
Post by: Bored chemist on 05/05/2013 14:18:18
Then a balloon full of ordinary air would float.
Of course, the krypton might be a bit expensive and the voices at the karaoke would be a bit off key.
Post by: CliffordK on 05/05/2013 18:03:52
Post by: damocles on 05/05/2013 23:35:50
However let us get down to basic issues. Pure radon is around 8 times as heavy as air, so that if we were to use pure radon we would need to be operating at a temperature of at least 2200 K (Ideal gas equation). Clearly this is quite impractical!
At 10% radon we would be needing to achieve a lift factor of about 10, which would mean a working temperature above 500 K. I seriously doubt that the radon would give off enough energy in its radioactive decay to achieve that.
Post by: cheryl j on 06/05/2013 02:42:13
Hold your party in a sealed chamber containing 20% oxygen mixed with something very dense. Argon might just do it but lets go with krypton to make sure.
Then a balloon full of ordinary air would float.
Of course, the krypton might be a bit expensive and the voices at the karaoke would be a bit off key.
Man, chemists must have weird birthday parties. I can just imagine what the clowns look like!
Post by: CliffordK on 06/05/2013 05:49:56
So radium is too heavy.
There are several isotopes of Argon (http://en.wikipedia.org/wiki/Isotopes_of_argon) that have half-lives in from a few minutes, to a few hours to a month to 269 years.
Neon also has radioactive isotopes (http://en.wikipedia.org/wiki/Isotopes_of_neon), but the longest half-life is 3.38 minutes, and thus one's balloon may not stay aloft for long if it was using radioactive Neon as a heat source.
I got it!!!!!!!
I thought it was interesting that H2 Hydrogen was only marginally more buoyant (at sea level) than Helium despite having half the molecular weight.
What about filling one's balloon with pure Tritium. With a molecular weight of 6, it would be slightly more dense than Helium (MW 4). However, perhaps it would generate a little energy to make up for the excess weight. And the decay product, 3He is still a lighter than air gas.
Post by: damocles on 06/05/2013 08:02:13
Quote
What about filling one's balloon with pure Tritium. With a molecular weight of 6, it would be slightly more dense than Helium (MW 4). However, perhaps it would generate a little energy to make up for the excess weight. And the decay product, 3He is still a lighter than air gas.
Yes Clifford, tritium would work. Of course apart from being explosively flammable, prohibitively expensive, and highly toxic, tritium is not a great source of energy -- a beta emitter with a particle energy of only 18 keV. Its half life of 12.26 yr would mean that it is only emitting at a few microwatt of power per mole.
Post by: Bored chemist on 06/05/2013 09:41:34
How hot it will get depends on the size of the balloon because it is heated in proportion to the volume (which varies as the cube of the radius) but loses heat in proportion to the area (which varies as the square of the radius).
The other factor is how long it takes to reach thermal equilibrium compared to how long it takes for all the radioactive material to decay.
In the limit, at the centre of the balloon the heat can't escape because it's surrounded by other gas which is also "trying to cool down".
At that point the temperature will keep rising until the heat liberated by the decay is absorbed by the decay products. The heat capacity of the products will be calculable as 1/2 k per degree of freedom.
The energy released depends on the radioisotope.
Lets consider tritium.
It decays with an energy of about 18KeV but a lot of that is carried off by the antineutrino and never gets seen again (to a fair approximation) leaving, on average 5.7KeV behind to share out among the debris.
The products (once they get really hot) are the He3 nucleus and 2 electrons so that's 3 particles.
Each can move in any of 3 directions so that's 3 degrees of freedom. They are too symmetrical to vibrate or rotate.
So,
K is about 10^-5 eV per k
and we have 3 particles with 3 degrees of freedom so that's a heat capacity of 9/2 k per tritium and the energy is about 5700 eV
So the temperature will rise to something like a hundred million Kelvin.
At which point, I think, the remaining T3 and H3 will start to fuse and your balloon will turn into a star.
The party will go with a bang.
Slightly more sensibly, if you used one of the stable isotopes of neon, the balloon would float if it was big enough.