Post by: talanum1 on 21/08/2020 13:57:15
-4*H*e^{-r/r_0}/r^2 = m_p*v^2*r.
By my model, the L of this nucleon must equal ħ, so:
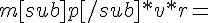 ħ
ħSolving these two equations for v we get a value for v of more than the speed of light. What am I doing wrong?
What is wring with this websites Tex?
Post by: Bored chemist on 21/08/2020 14:31:08
What am I doing wrong?Probably this.
By my model
Not defining "L" may also be a factor.
Post by: talanum1 on 21/08/2020 14:56:25
Probably this.
Quote from: talanum1 on Today at 13:57:15
By my model
It can't be my model. Other models require Orbital Angular Momentum of Boron (5 protons, 5 neutrons) to be 3ħ.
Post by: Bored chemist on 21/08/2020 15:34:04
Because that's not new.
"Spin" isn't spin.
Post by: talanum1 on 21/08/2020 15:47:17
Are you saying that the tangential speed of a nucleus exceeds C?
Because that's not new.
Yes, by orders of magnitude. Is it accepted?
Post by: Bored chemist on 21/08/2020 17:17:01
Yes, for at least 30 years that I know of in the case of electrons and (I think) protons.Are you saying that the tangential speed of a nucleus exceeds C?
Because that's not new.
Yes, by orders of magnitude. Is it accepted?
Post by: Kryptid on 21/08/2020 17:28:24
Post by: talanum1 on 21/08/2020 17:41:00
Where did you get your equations from?
From the internet and a physics book.
They predict speeds of order 10^32 m/s !
Post by: Kryptid on 21/08/2020 17:44:09
Post by: talanum1 on 21/08/2020 17:54:23
Post by: Kryptid on 21/08/2020 17:56:04
Post by: alancalverd on 21/08/2020 18:55:32
Post by: evan_au on 22/08/2020 02:00:19
Quote from: OP
What is wring with this websites Tex?For formatting maths equations on the forum, see: https://www.thenakedscientists.com/forum/index.php?topic=45718.msg397742#msg397742
But for simple equations, the editing tools with Greek letters and subscript/superscript icons work pretty well.
Quote from: OP
Solving these two equations for v we get a value for v of more than the speed of light. What am I doing wrong?At the subatomic scale, particles don't have a definite position, velocity, or momentum at a particular time.
- All these things become a bit "fuzzy", and particles behave like waves (and vice-versa).
- This is especially apparent with lighter particles, like the photon and electron, but still true of more massive particles like protons and quarks
- Quantum theory provides a very accurate picture of the world, but theoreticians differ in how they interpret "why" it gives these accurate results
- One view is that quantum theory estimates the probability of finding a subatomic particle in a particular position (if you try to measure it). But it gives you no idea of where the particle is between measurements - it could be almost anywhere. So you can't really calculate a "velocity" for the particle.
- This is summarized in Heisenberg's uncertainty principle:
See: https://en.wikipedia.org/wiki/Uncertainty_principle
Post by: talanum1 on 22/08/2020 13:20:06
I think one of the problems is that you are speaking of centripetal force. Quantum scale objects don't orbit each other in the way that planets and moons do. Centripetal force need not apply.
In my model we have a nucleon orbiting others in a circular orbit. It is the same concept: an object with mass orbiting in a forcefield. So why shouldn't centripetal force apply. See figure for Boron with 4 neutrons:
[ Invalid Attachment ]
If space breaks down at this scale, centripetal force would too, but we know breakdown happens at a much lower scale.
Orbital angular momentum doesn't mean the same in quantum physics as it does for macroscopic objects.
The formula is the same.
At the subatomic scale, particles don't have a definite position, velocity, or momentum at a particular time.
What formula do I use then? I have a textbook calculating the energy levels of the Hydrogen atom (sub-atomic) using particles, forces and velocities. Why should something similar not work at the nuclear level?
Post by: Kryptid on 22/08/2020 17:30:48
In my model we have a nucleon orbiting others in a circular orbit.
Then your model is wrong because particles don't behave that way. The fact that you calculated them to move faster than light is just more evidence that your model doesn't work.
Post by: alancalverd on 22/08/2020 20:36:28
In my model we have a nucleon orbiting others in a circular orbit.I think an infinitesimally small monkey juggling red (proton) and white (neutron) balls is a more realistic model.
Quote
What formula do I use then? I have a textbook calculating the energy levels of the Hydrogen atom (sub-atomic) using particles, forces and velocities. Why should something similar not work at the nuclear level?A sound philosophical question. The scientific question, however is (a) why should it and (b) why doesn't it? And what, pray, is a sub-atomic atom?
Post by: Kryptid on 22/08/2020 21:52:56
Post by: talanum1 on 24/08/2020 13:10:07
The measured values for energy levels also gives faster than light speeds.
Post by: talanum1 on 31/08/2020 10:53:31
If you do insist on using a centripetal force equation, try finding a relativistic one instead of a classical one.
I used it, but γ cancells.
Post by: Bored chemist on 31/08/2020 11:03:23
If you show your working, someone might be able to spot errors in it.If you do insist on using a centripetal force equation, try finding a relativistic one instead of a classical one.
I used it, but γ cancells.
Post by: talanum1 on 01/09/2020 14:05:34
[ Invalid Attachment ]
Post by: Bored chemist on 01/09/2020 16:50:37
Centripetal forces don't apply.