Post by: thedoc on 17/09/2015 19:50:02
About the uncertainty principle (as distinct from the observer effect) - according to the principle, is it the case that a) an attempt to determine the position of a particle changes the determinabiity of the momentum of the particle (in some kind of inverse proportion) and vice versa, or b) there is in pre-existence a degree of determinability of a particle's position and momentum (in inverse proportion) which observation determines but does not change. or c) other? If you could answer this question for me, could you please render it comprehensible to a relative simpleton such as I?
What do you think?
Post by: Bill S on 17/09/2015 21:45:23
Post by: Atomic-S on 18/09/2015 07:15:30
Quote
an attempt to determine the position of a particle changes the determinabiity of the momentum of the particle (in some kind of inverse proportion) and vice versaWithin my understanding, this view comes closer to the truth than the other; however everything surrounding this matter is conceptually problematic. I think, however, that the following is applicable:
If you study the mathematical relationships between position and momentum at the quantum level, you will find that the relationship between their wave functions is exactly a Fourier transform. Uncertainty is inherent in Fourier transforms by definition. If a signal has an exact frequency, then its time of occurrence is indeterminate, and vice versa. A signal that occurs within a limited time interval has a spectrum of frequencies of nonzero width, and thus cannot be said to have "a" frequency. It is impossible for a signal to have both an exact time of occurrence and an exact frequency, by definition of the terms "time of occurrence" and "frequency". This uncertainty is not an outgrowth of inaccurate measurements but the very definitions of the quantities. However, if observational circumstances are such that the shortest time interval of interest and the narrowest frequency interval of interest are both larger than some product, we can speak of the signal as having, for practical purposes, both a definite frequency and a definite time of occurrence.
These concepts presumably apply to quantum mechanics. There are difficulties, however, when trying to do so, as follows: If we accept the Fourier transform as the correct description of position and momentum, then the correct statement concerning position and momentum is that a particle in general has neither, but only its wave function. Thus, as in the case of the signal described above, the particle is correctly described by, and only by, its wave function. But here is where things get difficult: we have no way of observing the wave function directly, but are limited to observing its observable consequences. The apparatus with which we observe these consequences is likewise composed of wave functions, none of which we can directly observe. It would appear that the uncertainty principle (in the sense that the outcome of a quantum process cannot in general be predicted, but that the probability of possible outcomes can be, based on the square of the wave function) must have something to do with this observational constraint. Not the erratic path of the particle, but the inherent limits imposed by the order of things, is the source of our problem and limits us to dealing in probabilities. If this is the correct understanding, then an attendant conclusion seems to be that at the quantum level, no experiment can be truly replicated. Thus in, for example, the double slit experiment, every time the experiment is done, the screen is in a different quantum state; and there is no way to guarantee that its state, as relates to that of the particle in any one iteration of the experiment, will exactly match the way the states relate in a different iteration, and that remains true even at absolute zero of temperature. If that is so, it is sufficient to account for the fact that quantum experiments produce outcomes that do not duplicate themselves reliably.
Post by: guest39538 on 18/09/2015 10:08:06
John Davies> asked the Naked Scientists:
About the uncertainty principle (as distinct from the observer effect) - according to the principle, is it the case that a) an attempt to determine the position of a particle changes the determinabiity of the momentum of the particle (in some kind of inverse proportion) and vice versa, or b) there is in pre-existence a degree of determinability of a particle's position and momentum (in inverse proportion) which observation determines but does not change. or c) other? If you could answer this question for me, could you please render it comprehensible to a relative simpleton such as I?
What do you think?
Random velocity
Post by: mathew_orman on 18/09/2015 10:55:28
John Davies> asked the Naked Scientists:This principle has a domain of it's own called QM...
About the uncertainty principle (as distinct from the observer effect) - according to the principle, is it the case that a) an attempt to determine the position of a particle changes the determinabiity of the momentum of the particle (in some kind of inverse proportion) and vice versa, or b) there is in pre-existence a degree of determinability of a particle's position and momentum (in inverse proportion) which observation determines but does not change. or c) other? If you could answer this question for me, could you please render it comprehensible to a relative simpleton such as I?
What do you think?
According to R.Feynman, logical thinking is forbidden or incompatible with QM and QED...
Post by: mathew_orman on 18/09/2015 11:00:43
Post by: lightarrow on 18/09/2015 13:25:27
John Davies> asked the Naked Scientists:It depends on what exactly you mean with "determinabiity": every term in physics has its own technical meaning and definition.
About the uncertainty principle (as distinct from the observer effect) - according to the principle, is it the case that a) an attempt to determine the position of a particle changes the determinabiity of the momentum of the particle (in some kind of inverse proportion) and vice versa, or b) there is in pre-existence a degree of determinability of a particle's position and momentum (in inverse proportion) which observation determines but does not change. or c) other? If you could answer this question for me, could you please render it comprehensible to a relative simpleton such as I?
What do you think?
Having said this, the answer that seems nearer to what I think you mean with that term is a).
Explanation: a measurement can change the "state" of the particle; for example, if before the measurement the particle had a quite well determined momentum, after a precise measurement of position the particle won't be in such a state anylonger, so its momentum becomes very INdetermined: it means that a measurement of momentum will give a value p1, you repet the experiment with another particle prepared EXACTLY in the same way as the first and you will find its momentum is p2, etc. After a great number of equal experiments, you will find the momentum values are greatly "spread".
It's this great statistical "spread" the meaning of "particle with momentum very uncertain".
The Δp of the uncertainty principle is exactle the standard deviation of the distribution of the momentums.
Analogous reasoning for Δx
--
lightarrow
Post by: Bill S on 18/09/2015 15:23:00
One could interpret a) as saying that the particle had a certain position and momentum before the measurement was made, but that one of these was disrupted by measurement of the other. Surely, this is not what the uncertainty principle is about.
Post by: PmbPhy on 18/09/2015 19:28:57
Quote from: John Davies
It's "c," none of the above. When the position of a particle is measured the system collapses into a position eigenstate. When this happens the momentum is totally undefined. The uncertainty principle for position and momentum (there are many other uncertainty relations) is a relationship between standard deviation of position measurements to the standard deviation of momentum measurements. It really doesn't apply to single measurements but to an ensemble of measurements.
About the uncertainty principle (as distinct from the observer effect) - according to the principle, is it the case that a) an attempt to determine the position of a particle changes the determinabiity of the momentum of the particle (in some kind of inverse proportion) and vice versa, or b) there is in pre-existence a degree of determinability of a particle's position and momentum (in inverse proportion) which observation determines but does not change. or c) other? If you could answer this question for me, could you please render it comprehensible to a relative simpleton such as I?
What do you think?
See: https://en.wikipedia.org/wiki/Uncertainty_principle
Post by: Mordeth on 19/09/2015 05:21:12
I will do my best to describe the equations as simply as possible, as a more rigorous derivation would probably derail the thread.
The change or uncertainty (Δ) of momentum (p) of a particle is described by Planck's constant (h) divided by the de Broglie wavelength ( λ ). So Δp = h/λ
The change or uncertainty (Δ) in position (x) of a particle is simply its wavelength (λ). So Δx = λ
So we can now substitute Δx above in the momentum equation where we had λ. So now Δp = h/Δx
We can rewrite this is ΔpΔx = h.
It was rewritten more precisely as ΔpΔx ≥ h/2π
Other than E = mc², what you see above is the most famous equation in physics. What it tells you is that
if we decrease the uncertainty of one variable, we must automatically increase the uncertainty of the other in order to satisfy the inequality of the equation. So the more we measure momentum, which decreases Δp, the less we will know about position, as Δx must then increase, and vice versa. Put another way, the error in momentum times the error in position must always be greater than Planck's constant. Therefore, to know for certain one variable will cause the other to be unknown by definition.
Post by: PmbPhy on 19/09/2015 07:09:59
Quote from: Mordeth
Particles are probability waves, and are described by mathematical relationships.That is incorrect. Particles are not probability waves. All that you can say about particles is that when their position is measured they are found at a single point in space and not spread out in space. A particle cannot be said to have a location. All that can be said about them is that for each location there's a certain probability of finding the particle in a region centered at that place in space. The size of that region comes into the probability calculation too.
Quote from: Mordeth
We can calculate, by measurement, certain properties regarding particles.I don't understand this. What does "We can calculate, by measurement, .." mean?
Quote from: Mordeth
The change or uncertainty (Δ) of momentum (p) of a particle is described by Planck's constant (h) divided by the de Broglie wavelength ( λ ). So Δp = h/λI'm sorry to inform you that you have this all wrong. No worries though. I'm here to help! :) Most people who haven't taken an advanced course on quantum mechanics often get this wrong. So be glad that your mistake has been found and as such you're now in a position to learn it correctly! :)
The change or uncertainty (Δ) in position (x) of a particle is simply its wavelength (λ). So Δx = λ
So we can now substitute Δx above in the momentum equation where we had λ. So now Δp = h/Δx
We can rewrite this is ΔpΔx = h.
It was rewritten more precisely as ΔpΔx ≥ h/2π
Go to: https://en.wikipedia.org/wiki/Uncertainty_principle#Robertson.E2.80.93Schr.C3.B6dinger_uncertainty_relations
"The change ... (Δ)" is a wrong statement. That symbol does not stand for change. It refers to standard deviation. Check https://en.wikipedia.org/wiki/Uncertainty_principle again and you'll understand it better. The "uncertainty" of, for example, position, cannot be determined simply by its wavelength and uncertainty of momentum also isn't determined by h over wavelength. The uncertainty of an observable is a function of the wave function. You simply cannot determine the uncertainty of something without knowing the wave function. Once you know the wave function you can then determine the uncertainty of any observable. If you want to measure the uncertainty rather than calculate it then you need to run the same experiment a large number of times starting from the same exact state of the wave function. That's how it can be a standard deviation. You simply cannot determine a standard deviation of a single experiment. The uncertainty is determined by calculating an integral.
To see an example look at my old website. The wave function that I used was a Gaussian function.
http://home.comcast.net/~peter.m.brown/qm/x_square.htm
Other than E = mc², what you see above is the most famous equation in physics. What it tells you is that
if we decrease the uncertainty of one variable, we must automatically increase the uncertainty of the other in order to satisfy the inequality of the equation. So the more we measure momentum, which decreases Δp, the less we will know about position, as Δx must then increase, and vice versa. Put another way, the error in momentum times the error in position must always be greater than Planck's constant. Therefore, to know for certain one variable will cause the other to be unknown by definition.
[/quote]
Post by: Mordeth on 19/09/2015 18:28:09
I understand the Uncertainty principle quite well, but thank you. What I provided was a simple derivation meant to explain it for the average person using math. It is not innacurate or wrong. Furthermore, the term uncertainty is commonly used to describe the delta of position and momentum when explaining the Uncertainty Principle to the average person. I can prove this if you like. You can start by reading here:
http://pdg.web.cern.ch/pdg/cpep/unc_vir.html
CERN themselves use this term, and I highly suggest you read the whole article.
Also see here:
http://www.relativitycalculator.com/Heisenberg_Uncertainty_Principle.shtml
Post by: PmbPhy on 19/09/2015 19:26:13
Quote from: Mordeth
Hi pmb,Nothing personal but I don't think you know how it's defined. The way you described it is incorrect, regardless of whether you were explaining it to an expert or to an amateur, the definition is the same. As you wrote the uncertainty it was independent of the wavefunction. However in quantum mechanics the uncertainty is a function of the wavefunction.
I understand the Uncertainty principle quite well, but thank you. What I provided was a simple derivation meant to explain it for the average person using math. It is not innacurate or wrong.
Quote from: Mordeth
Furthermore, the term uncertainty is commonly used to describe the delta of position and momentum when explaining the Uncertainty Principle to the average person.To explain it correctly one has to explain that the uncertainty is the standard deviation of a set of measurement data. I.e. it's defined by
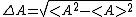
where:
<A>2 =
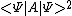
<A2> =
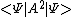
Quote from: Mordeth
I can prove this if you like.I don't understand. What is it that you're saying that you can prove?
Quote from: Mordeth
You can start by reading here:That is merely a statement of the uncertainty principle and nothing more. And it's quite vague at that in that it doesn't explain that "the uncertainty in x" means "the standard deviation of x".
http://pdg.web.cern.ch/pdg/cpep/unc_vir.html
I don't understand. What is it that you're saying that you can prove?
Quote from: Mordeth
CERN themselves use this term, and I highly suggest you read the whole article.What term? Are you trying to say that I don't understand what uncertainty is or what the uncertainty principle is? If so then it's exactly as stated in the Wikipedia link that I gave above.
Also see here:
http://www.relativitycalculator.com/Heisenberg_Uncertainty_Principle.shtml
In this link the uncertainty relation isn't derived rigorously but uses a particular example of the double slit experiment. If you understand what's derived on that page then you'd know that the probability is a standard deviation. That's how its related to the wave function.
If I gave you a wave function would you know how to determine the uncertainty in x from it?
Post by: Mordeth on 20/09/2015 01:53:06
The standard deviation reflects the spread or expected fluctuations in a series of measurements of an observable in a given state. This spread in QM is the uncertainty, which grows for one operator as the other shrinks. The standard deviation is commonly referred to as the "uncertainty" in this context. In fact, the standard deviation is what is describing the uncertainty. I am not sure why this is so difficult for you to understand.
Post by: Franklin_Uhuru on 20/09/2015 03:24:07
Quote
I am not sure why this is so difficult for you to understand.
I don't "understamd" it either.
You are trying to use 19th century physics to explain 21st century phenomena. People - in order to pass Freshman physics need to understand that sub-atomic particles exist as clouds of probability and not discrete points of matter.
I have never heard "standard deviation" applied to either the Dirac or Heisenberg treatments of probability densities. Nor is standard deviation used used to explain the hyperfine sprectum of Hydrogen.
Post by: PmbPhy on 20/09/2015 06:07:36
Hi pmb,Why are you claiming that I'm having a difficult time understanding it? From the beginning I've been saying that the standard deviation of an observable is the uncertainty of that variable. I've said that from my very first post in this thread.
The standard deviation reflects the spread or expected fluctuations in a series of measurements of an observable in a given state. This spread in QM is the uncertainty, which grows for one operator as the other shrinks. The standard deviation is commonly referred to as the "uncertainty" in this context. In fact, the standard deviation is what is describing the uncertainty. I am not sure why this is so difficult for you to understand.
In my very first post in this thread I wrote
Quote
The uncertainty principle for position and momentum (there are many other uncertainty relations) is a relationship between standard deviation of position measurements to the standard deviation of momentum measurements.It was you who in your very first post claimed that So Δp = h/λ which means that the uncertainty was a function of wavelength only and not a function of the wavefunction.
In another post I wrote, .after you claimed "The change ... (Δ)"
Quote
"The change ... (Δ)" is a wrong statement. That symbol does not stand for change. It refers to standard deviation.
Post by: PmbPhy on 20/09/2015 06:17:00
Quote from: Franklin_Uhuru
I have never heard "standard deviation" applied to either the Dirac or Heisenberg treatments of probability densities. Nor is standard deviation used used to explain the hyperfine sprectum of Hydrogen.Then you haven't taken an advanced course in QM yet. If/when you do you'll find that the uncertainty in an observable is the standard deviation of a large set of measurements of that observable. The system is placed in the state psi and the observable measured. The system is then placed in the state psi and repeated. This is done a large number of times. The standard deviation is then calculated.
See - https://en.wikipedia.org/wiki/Uncertainty_principle
Quote
Thus, the uncertainty principle actually states a fundamental property of quantum systems, and is not a statement about the observational success of current technology.
Look in Introduction to Quantum Mechanics by David Griffiths at http://bookos-z1.org/book/2031469/90ac0b
Search for standard deviation and uncertainty.
Post by: alancalverd on 20/09/2015 10:52:58
Here's the difference
If you want to know the speed of a macroscopic entity like a car, you can bounce a radar pulse off it and measure the doppler shift. But the return pulse will involve a lot of noise, so you need to average it over several cycles. So you can end up with a very precise value for the average speed of a car over several meters, but no information about its speed at any particular point in that zone. That's uncertainty.
The hydrogen atom consists of an electron and a proton. We don't care about the position or momentum of the electron, but it's pretty clear that the atom has a nonzero radius that is a heck of a lot bigger than the radius of a proton. Why? Because the position of the electron is inherently indeterminate.
The joy of the indeterminacy concept is that it permits modelling of complex molecules and collaborative effets such as conduction , semiconduction and superconduction, without resort to artificial "virtual measurements" and the like.
I agree with FU (and Heisenberg and Feynman) on one point here: there's nothing to be gained by starting with a Bohr atom nowadays.
Post by: lightarrow on 20/09/2015 10:59:50
What I don't understand is why textbooks (not those which do things more seriously) don't write this simple phrase!
--
lightarrow
Post by: PmbPhy on 20/09/2015 15:31:33
Quote from: Mordeth
The change or uncertainty (Δ) of momentum (p) of a particle is described by Planck's constant (h) divided by the de Broglie wavelength ( λ ). So Δp = h/λAt this point I'd like to point out the errors above. The first part implies that the standard deviation of the x-component of momentum, i.e. Δpx, is exactly the same for all wavefunctions and has the value h/λ while the second part implies that the standard deviation of the x-component of position, i.e. Δx, is exactly the same for all wavefunctions and has the value Δx = λ. In reality Δp and Δx are completely determined by the wavefunction
The change or uncertainty (Δ) in position (x) of a particle is simply its wavelength (λ). So Δx = λ
So we can now substitute Δx above in the momentum equation where we had λ. So now Δp = h/Δx
We can rewrite this is ΔpΔx = h.
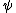 .
.
Post by: Craig W. Thomson on 20/09/2015 17:29:32
John Davies> asked the Naked Scientists:I would like to take a stab at this one as a layman. I'm adapting an analogy I've used before which is not perfect, but should prove useful.
About the uncertainty principle (as distinct from the observer effect) - according to the principle, is it the case that a) an attempt to determine the position of a particle changes the determinabiity of the momentum of the particle (in some kind of inverse proportion) and vice versa, or b) there is in pre-existence a degree of determinability of a particle's position and momentum (in inverse proportion) which observation determines but does not change. or c) other? If you could answer this question for me, could you please render it comprehensible to a relative simpleton such as I?
What do you think?
Imagine there's a two-bladed spinning propeller on an airplane. You want to determine the momentum and position of the propeller's blades, but the only technique you have available is to fire objects at it.
First, fire a small bullet out of a gun at the propeller. It keeps spinning, barely affected by the bullet, but the bullet ricochets off the propeller. By performing measurements on the bullet after the ricochet, you could work out something about the propeller's speed, but you couldn't deduce much about which blade of the propeller caused the ricochet, or in other words, what position (location of both blades) the propeller was in when the collision occured.
Now, try firing a large cannonball at the propeller. This knocks one of the blades clean off. You can tell exactly where that happened (which blade it happened to), but you can't determine how fast the propeller is spinning because it was destroyed in the collision and no longer spins.
I'm trying to think of something in between those two extremes. Maybe a baseball-sized rock launched from a pitching machine. In that case, the rock would bounce off, so you could deduce something about the propeller's momentum from the rock's trajectory, but it was also large enough to damage the propeller so that it spins at a different rate now. So, you can determine less about the propeller's position from the rock than the cannonball, but also less about its momentum than with a bullet.
Yes, I know this analogy has some serious problems. This is merely an illustration to help convey an idea.
Post by: lightarrow on 21/09/2015 10:31:18
I would like to take a stab at this one as a layman. I'm adapting an analogy I've used before which is not perfect, but should prove useful.But I'd call it a "metaphor", more than "analogy".
Imagine there's a two-bladed spinning propeller on an airplane. You want to determine the momentum and position of the propeller's blades, but the only technique you have available is to fire objects at it.
First, fire a small bullet out of a gun at the propeller. It keeps spinning, barely affected by the bullet, but the bullet ricochets off the propeller. By performing measurements on the bullet after the ricochet, you could work out something about the propeller's speed, but you couldn't deduce much about which blade of the propeller caused the ricochet, or in other words, what position (location of both blades) the propeller was in when the collision occured.
Now, try firing a large cannonball at the propeller. This knocks one of the blades clean off. You can tell exactly where that happened (which blade it happened to), but you can't determine how fast the propeller is spinning because it was destroyed in the collision and no longer spins.
I'm trying to think of something in between those two extremes. Maybe a baseball-sized rock launched from a pitching machine. In that case, the rock would bounce off, so you could deduce something about the propeller's momentum from the rock's trajectory, but it was also large enough to damage the propeller so that it spins at a different rate now. So, you can determine less about the propeller's position from the rock than the cannonball, but also less about its momentum than with a bullet.
Yes, I know this analogy has some serious problems. This is merely an illustration to help convey an idea.
You have written that it's not perfect but I would say it's even less [;)].
Remember, for example (but it's not the only thing), what I and Pete remarked about the statistical meaning of the uncertainty on an observable's measurement.
--
lightarrow