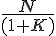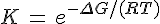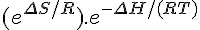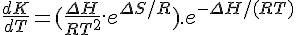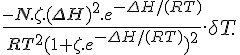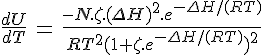Post by: chiralSPO on 24/05/2022 18:32:34
Please, don’t sidetrack this discussion with comments about alternative technologies, or putting on a sweater, or the pros/cons of a certain temperature, or anything about climate change. I want to keep the discussion focused on the chemistry/physics/engineering/math of optimizing a system as described in the next post (the remainder of this post is all background info pertaining to how I got to the question at hand—feel free to skip ahead)
A significant portion of our society’s energy usage goes towards heating and cooling our homes and places of business. There are many ways of passively controlling temperature inside building including insulation and reflective vs light-absorbing exteriors etc. But I want to focus this thread on materials/systems that have a mediating influence on temperature by virtue of their heat capacity (I believe “thermal mass” is the term used by architects).
Some substances have an extremely high heat capacity per unit volume. For example, liquid water; coming in at about 4.2 kJ•L–1 •K–1, with some minor variation across its liquid range. But this pales in comparison to the incredible latent heat of a phase change.
For example, t-butanol melts/freezes at 298 K (25 °C, or 77 °F), and has a latent heat of fusion of about 116 kJ•L–1 •K–1. Any room at equilibrium with a bottle of t-butanol would have a significant “thermal mass” crossing 298 K (25 °C, or 77 °F). A sufficiently large bottle of liquid t-butanol, with sufficiently fast heat exchange to the room would effectively prevent the room from dropping below 25 °C. Likewise, if the t-butanol were solid, the room could be held below this temperature. If you want a different temperature, you can pick a different substance, which will have a different melting point (and different latent heat of fusion)
The problem with this approach (ignoring issues specific to the substance of choice), is that it only works for a single temperature (very narrow temperature range). It probably wouldn’t be economically feasible to have such a large reservoir of this substance with heat exchangers etc. necessary to keep a room (or building) at a single temperature. And if the temperature at any point drifted far from the melting point, then the thermal mass of the substance would be very small compared to the latent heat of fusion.
One possible solution (no pun intended) would be to have a few different reservoirs containing different substances, with different critical temperatures. For example dmso melts at 19 °C (66 °F). So one could imagine a room that is kept between 19 °C and 25 °C with one reservoir of t-butanol and one of dmso. Or one could imagine using only one solvent, but with two (or more) reservoirs with different amounts of solute, resulting in slightly different melting points (by virtue of colligative properties).
Post by: chiralSPO on 24/05/2022 18:33:25
Imagine a chemical reaction in equilibrium. For simplicity, we will just say there are two states, A and Z.
A
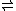 Z
ZΔGforward = ΔHforward – T• ΔSforward
For this reaction to work, entropy and enthalpy must be opposing each other ΔHforward and ΔSforward must both be positive (so as temperature increases, an endothermic reaction goes forward). Therefore, ΔHreverse and ΔSreverse must both be negative.
As the temperature changes, the equilibrium concentrations of the product/reactant states changes, absorbing heat as the temperature increases, and releasing heat as it decreases.
ΔG = –RTln([Z]/[A])
or
ΔH – T ΔS = –RTln([Z]/[A])
Some rearranging gives:
ΔH = T(ΔS – R ln([Z]/[A]))
In the real world, both ΔH and ΔS are also both functions of T, but let’s hold off on that for now, unless someone can provide a good reason why it must be considered. (I think it should be pretty subtle across the ranges of T we are trying to cover).
We can imagine the enthalpy of the reaction (ΔH) is what we are able to select. The more positive it is, the more energy is absorbed per molecule that is transformed from A to Z (and more released when going from Z to A). But if the equilibrium lies too far to one side, we will be limited in how much energy can be handled in one direction. (ie if 99.999% is in state A at a given temperature, then only a tiny amount of energy can possibly be released when the temperature falls). If we want the system to be able to both absorb and release significant amount of heat at a comfortable temperature, the larger the ΔH term is, the larger the ΔS term must be as well (so that T• ΔS is close to ΔH when [A] is close to [Z].
So the question becomes. For a given high and low temperatures (Thigh and Tlow), what is the optimal choice of ΔH and ΔS, such that the system is most stable between those to temperatures?[/i]
My algebra and calculus is a bit rusty. So, while my intuition indicates that there will be a very easy way to express these trade-offs mathematically, and find the optimum values, I have not been able to convince myself that the answers I am finding are correct.
Post by: Halc on 24/05/2022 19:19:40
But this pales in comparison to the incredible latent heat of a phase change.I don't get it. Suppose I have a material that melts/freezes at room temperature. This only works once, and then it's done. Say I want to heat my building in the winter. I have liquid 'stuff' that freezes as the room temp drops just below where I want it, so it keeps the room warm until it's entirely frozen. Now what? How am I going to get it into liquid state again? I have to turn the heater on and it has all the much more work to do since it has to melt all this nice stuff on top of actually heating the place. It seems I've saved no energy at all, so I'm not sure what you're getting at.
Heating/cooling is all about insulation, not thermal capacity. The more thermal energy that passes from the hot side to the cold side, the more energy it takes to put it back.
Industry, the primary consumer of resources, seems not to care. In the middle of winter I watched the power consumed by the air conditioners in the computer lab. All it needed was a fresh air fan on the roof since it was well below freezing outside, and there they are pumping heat out of the lab to the radiator on the roof, and not even into the heating system keeping the offices warm.
Another building (built for IBM) had the heater break down on an August day. We had the doors/windows open and still had to wear winter coats because there was no heat to mix with the cold system. Temp was set just like water in houses: by mixing just the right amount of hot and cold, and not just turning off the whole system when it was cool enough. Apparently the utility bill was of no concern.
Post by: chiralSPO on 24/05/2022 19:47:57
I don't get it. Suppose I have a material that melts/freezes at room temperature. This only works once, and then it's done. Say I want to heat my building in the winter. I have liquid 'stuff' that freezes as the room temp drops just below where I want it, so it keeps the room warm until it's entirely frozen. Now what? How am I going to get it into liquid state again? I have to turn the heater on and it has all the much more work to do since it has to melt all this nice stuff on top of actually heating the place. It seems I've saved no energy at all, so I'm not sure what you're getting at.Sure, the use of a phase-change material would not solve this particular problem. Imagine a climate (or time of year), when the temperature changes throughout the day, oscillating between a low of 15 °C and a high of 30 °C (≈59 °F to ≈86 °F), and you would rather keep the house at 22 °C (≈72.5 °F) with minimal energy usage.
Heating/cooling is all about insulation, not thermal capacity. The more thermal energy that passes from the hot side to the cold side, the more energy it takes to put it back.
Industry, the primary consumer of resources, seems not to care. In the middle of winter I watched the power consumed by the air conditioners in the computer lab. All it needed was a fresh air fan on the roof since it was well below freezing outside, and there they are pumping heat out of the lab to the radiator on the roof, and not even into the heating system keeping the offices warm.They should care. But, I would like to remind folks that the point of this thread is not to answer problems of economics, politics, or even practicality. I want to know how to express the relationships between ΔH and ΔS and working temperature range of such an equilibrium. Simple thermodynamics/algebra/calculus.
Another building (built for IBM) had the heater break down on an August day. We had the doors/windows open and still had to wear winter coats because there was no heat to mix with the cold system. Temp was set just like water in houses: by mixing just the right amount of hot and cold, and not just turning off the whole system when it was cool enough. Apparently the utility bill was of no concern.
Post by: evan_au on 24/05/2022 23:11:59
Quote from: Halc
I don't get it.Another on the sideline of practicality: It could make sense in a desert, where it gets well above 30C almost every day (including winter), and well below 15C almost every night (even in summer).
You would need two tubs of your phase change material which can be selectively exposed to outside air or inside air.
- During the day, you expose one tub to the outside air to heat it, while the other (cold) tub is exposed to inside air to keep the house cool.
- Reverse it overnight: Expose the hot tub to the inside to heat the house, while the other tub is exposed to the outside air to cool it, ready for the next day.
Effectively, you want a system that will produce a 12-hour phase change* in the outside temperature cycle.
* This is a temporal phase change, achieved by a physical phase change
For it to work well, your house would need to be well-insulated, and you would need fans to circulate air past the tubs of hot/cold materials.
Post by: chiralSPO on 25/05/2022 03:19:48
Effectively, you want a system that will produce a 12-hour phase change* in the outside temperature cycle.I see what you did there :-)
* This is a temporal phase change, achieved by a physical phase change
Post by: paul cotter on 25/05/2022 12:40:33
Post by: Bored chemist on 25/05/2022 13:17:42
You would need two tubs of your phase change material which can be selectively exposed to outside air or inside air.Just make the walls out of tanks of the material.
The idea works, but the amount of stuff you need is huge unless you have really good insulation.
Post by: chiralSPO on 25/05/2022 23:03:16
I understand the original idea as using an arbitrarily large quantity of phase change material to store energy and release it only at a transition temperature. I see no problem in principle other than the vast quantity of material needed. If going for a chemical change process things become more difficult as a lot of processes that are thermodynamically favourable do not proceed for kinetic reasons. With exothermic processes there is a risk of positive feedback leading to a runaway.
Thankfully, any chemical equilibrium that satisfies the reversibility requirements stipulated are not going to be at risk of thermal runaway.
Also, while it is true that favorable thermodynamics does not require practical kinetics, for now, let's leave that wrinkle out. I am envisioning very simple, fast, and reversible reactions such as proton transfer reactions—the equilibrium of protonation states of weak acids and bases, and their conjugate bases and acids.
A-H + B
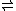 A– + B-H+
A– + B-H+One can think of controlling the ΔH by selecting A-H and B with appropriate relative proton affinities, and controlling the ΔS by selecting the ratio of A-H and B. In the extreme, we can think about one of them essentially being the solvent, for example a weak acid in water, (A-H +H2O
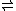 A– + H3O+) where the hotter it is, the more the acid dissociates, pushing the reaction to the right).
A– + H3O+) where the hotter it is, the more the acid dissociates, pushing the reaction to the right).But what I am most interested in at the moment, is just the math involved in figuring out how to maximize the ability of the solution to absorb/release energy over a desired temperature range.
Post by: Eternal Student on 25/05/2022 23:55:43
This question is inspiredWell, it is quite a good idea.
ΔG = –RTln([Z]/[A])Could you clarify this please? I'm not sure what your ΔG is, is it actually ΔG° ? Are [Z] and [A] concentrations at equillibirum only? i.d.k.
This is the conventional equation:
ΔG = RT Ln (Q/K)
Where ΔG = Gibbs free energy change for the system, (in the forward direction and at the specified concentrations).
Q = quotient of concentrations of products / reactants = [Z] / [A]
K = chemical equilibrium constant = Quotient as above but AT EQUILIBRIUM.
Just to clarify this, this ΔG is a function of 3 variables: The temperature, T, and the concentrations [Z] and [A].
[reference: https://chem.libretexts.org/Courses/Grand_Rapids_Community_College/CHM_120_-_Survey_of_General_Chemistry/7%3A_Equilibrium_and_Thermodynamics/7.11%3A_Gibbs_Free_Energy_and_Equilibrium ]
There seems to be a K missing in your expression, much as if you were assuming K always = 1.
This could be enough to stop your idea working completely. If K = 1 always, then the net reaction never shifts forward or backward - the equillibrium point remains with equal concentrations of products and reactants [Z] = [A] regardless of what happens. In this way it won't respond to changes in temperature at all.
To re-phrase this K ≠1. It is essential that K = K(T) = some function of temperature.
Using conventional theory, it seems that we can approximate K(T) = equillibrium constant at temperature T as
K(T) ≈ e -(ΔG°/ RT )
This quantity, ΔG° is not a function of the concentrations of the products and reactants. At most it is a function of the temperature, T, but more usually the temperature and pressure are also assumed to be standard temp. and pressure. Since you're interested in changes occurring around room temp. and pressure, it shouldn't be a problem to assume ΔG° is just a constant which you can find in a book for the reaction A → Z.
Anyway, re-arranging that equation we obtain: ΔG° = -RT Ln (K) = -RT Ln ([Z]/[A]) where [Z] and [A] are now only to be taken as the concentrations at equillibrium. That might have been the equation you were suggesting in your original post. It matters a lot because, if that was what you were doing, then when you re-arranged it to find ΔH I don't think it was the ΔH that you were actually hoping or thinking you'd find.
Summary: Sorry that was confusing. I'm confused and just trying to match up your notation with that used in some other texts on the subject. I need you to check or explain what it was you were hoping to suggest with your formula ΔG = -RT Ln ([Z] / [A]) .
Best Wishes.
Post by: alancalverd on 26/05/2022 06:56:43
As BC says, it does depend on having really good insulation but if you know your maximum insolation, minimum outdoor temperature, conductivity of your exterior cladding, and internal heat load (say 100W per person plus whatever other stuff you have in the building) you can calculate the mass of phase-change material required to keep the interior wall close to the melting point provided the night temperature is below your target for long enough.
And a practical thought: whilst heat storage systems have been proposed and implemented around melting, it's worth noting that the latent heat of vaporisation of water is about 7 times that of fusion, and the transition temperature can be relatively easily controlled by changing the pressure in the containment vessel. Worth looking at some more volatile materials?
Post by: Bored chemist on 26/05/2022 08:54:32
Post by: alancalverd on 26/05/2022 10:54:42
Or consider a sublimating solid?
Post by: chiralSPO on 26/05/2022 16:54:23
Could you clarify this please? I'm not sure what your ΔG is, is it actually ΔG° ? Are [Z] and [A] concentrations at equillibirum only? i.d.k.
Thank you for engaging my intended line of inquiry and trying to clarify points of confusion :-)
I am definitely not talking about ΔG° because I am trying to compare different temperatures and concentrations.
I think that [A] and [Z] are referring only to equilibrium concentrations (at each of the temperatures under consideration), so I am thinking about K, rather than Q. In the simplest case, A
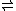 Z, Keq = [Z]/[A], though for an acid/base case (which I mentioned in another post), B– + A-H+
Z, Keq = [Z]/[A], though for an acid/base case (which I mentioned in another post), B– + A-H+ 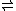 B-H + A, it could be Keq = ([B–][A-H+])/([B-H][A]). Another option would be a dissociation, A-B
B-H + A, it could be Keq = ([B–][A-H+])/([B-H][A]). Another option would be a dissociation, A-B 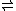 A+ + B–, where Keq = [A+][B–]/[A-B]
A+ + B–, where Keq = [A+][B–]/[A-B]
Post by: Bored chemist on 26/05/2022 19:43:30
But you can start with a tank of liquid and a fairly small head space. LV decreases with increasing pressure but may remain practicable up to 1000 bar or so depending on the critical point of the liquid.And you can watch the heat of vaporisation crash practically to zero (to exactly zero at T Crit.)
https://en.wikipedia.org/wiki/Enthalpy_of_vaporization
Post by: alancalverd on 26/05/2022 22:33:12
Post by: Bored chemist on 26/05/2022 23:44:36
All control systems have limits, which is why we need to use numbers to design them.Sometimes the number you need is "about a hundred or a thousand" which is the right ballpark for how much denser a solid is, compared to the vapour near atmospheric pressure.
Post by: Eternal Student on 27/05/2022 04:40:39
I've had another look at the mathematics and tried a few approaches. I've got something that looks reasonable but it's taking too long to write it down and mark up the equations with LaTex. I'll try and get it done but I'm off to the big city tomorrow and I know I won't be writing much here for at least a day.
The final results I can write down. They are dissapointing and probably only tell you what you should have already been able to guess. On the other hand... they make sense and that suggests I haven't gone too far wrong. Glass half-full or half-empty.
1. You want to have as large an amount of Substance A (or equivalently of substance Z) as possible. i.e. Whatever you do, do lots of it - make the biggest system you can.
2. You want a reaction with as large a ΔH as you can get. Infinity would be great but otherwise just as large as you can get.
3. What about ΔS? If you want maximum response from the system at room temperature (say about 300 Kelvin). Then you will need ΔS ≈ ΔH / 300. More generally the system will act most effectively as a buffer for the room temperature when ΔS = (whatever your value of ΔH is) divided by (whatever value of T you're trying to hold the room at). Changing the value of ΔS from that value has a rapid and detrimental effect: The system becomes almost non-changing (and hence unable to buffer the temperature) at your desired room temperature. Instead it works well as a buffer at a different temperature (which will be precisely T = ΔH / ΔS ).
You could have guessed these couldn't you? The mathematics will show it but it's going to be a day or more.
Best Wishes.
Post by: Bored chemist on 27/05/2022 09:00:21
And tables of melting points are easier to find than tables of ΔS
It's also worth thinking about the "units".
As Alan didn't notice, ΔH is typically given per mole or per unit mass.
On a practical basis you might want to look at the value per cubic metre.
And then you need to look at ΔH per £.
Post by: alancalverd on 27/05/2022 10:39:35
Sometimes the number you need is "about a hundred or a thousand" which is the right ballpark for how much denser a solid is, compared to the vapour near atmospheric pressure.Which is why I suggested using a liquid. Or a sublimating solid.
Post by: Bored chemist on 27/05/2022 13:36:43
Ok, because the vapour takes up an impractical amount of space you suggested turning a solid or liquid into the (impractical) vapour.Sometimes the number you need is "about a hundred or a thousand" which is the right ballpark for how much denser a solid is, compared to the vapour near atmospheric pressure.Which is why I suggested using a liquid. Or a sublimating solid.
Were you aiming to fail?
Post by: Eternal Student on 30/05/2022 15:25:22
I'll try and get it done but I'm off to the big city tomorrow and I know I won't be writing much here for at least a day.I'm really sorry but I'm not going to get this done any time soon.
I'm already on pages of work. Therefore no-one will want to read it, not even @chiralSPO .
I do not have the time to complete. I'm way behind on several other important tasks. The whole thing is now begining to cause me some stress and that's not how a forum should be.
I'm very sorry @chiralSPO but you should not wait for a detailed response from me, it would safer to assume it won't happen.
Apologies, Eternal Student.
Post by: chiralSPO on 31/05/2022 20:49:04
Hi again.I'll try and get it done but I'm off to the big city tomorrow and I know I won't be writing much here for at least a day.I'm really sorry but I'm not going to get this done any time soon.
I'm already on pages of work. Therefore no-one will want to read it, not even @chiralSPO .
I do not have the time to complete. I'm way behind on several other important tasks. The whole thing is now begining to cause me some stress and that's not how a forum should be.
I'm very sorry @chiralSPO but you should not wait for a detailed response from me, it would safer to assume it won't happen.
Apologies, Eternal Student.
Thanks for trying. I do not mean to give anybody homework to stress over.
I too have gotten bogged down in the math, which is why I reached out.
I have gotten somewhere:
Assuming a two state model (A
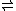 Z), the equilibrium ratio of concentrations is:
Z), the equilibrium ratio of concentrations is:[Z]T/[A]T = e–RT/(ΔΗ–TΔS)
Because we want ΔH–TΔS to be 0 at comfortable temperatures, that restricts the range of ΔH as a function of ΔS (or vice versa). The bigger ΔH is, the more thermal energy can be absorbed per mole of transformed molecule as T increases (and vice versa). But due to the exponential nature of the function providing [Z]/[A], there will be only a very narrow range in which there is an appreciable amount of both A and Z.
Because one is turning into the other, at a given temperature, T1, [Z]T1 = 1–[A]T1
KT1= (1–[A]T1)/[A]T1
And then at a different temperature, T2, KT2= (x+1–[A]T1)/([A]T1–x), where x is how many moles is getting converted.
amount of energy absorbed when going from T1 to T2 = ΔH•x
And then I get lost in the algebraic rearrangements when trying to get ΔH•x as a function of T1 and T2. (which then means I cannot use calc to optimize it).
I may keep banging my head against this wall for a while (hints or answers would still be appreciated). But I think the long and short of it is: there isn't really much advantage to trying for a gradual transition--it doesn't change the overall amount of energy that can be absorbed (ΔH). So, as Bored chemist says, maximizing ΔH/£ (or ΔH/$) for something with a comfortable equilibrium temperature is probably the way to go, and then just use as much as needed to keep the temp where you want it.
:)
Post by: Bored chemist on 31/05/2022 22:02:50
But we used some sort of thermostat to maintain the temperature near 20C .
You can get away with the equilibrium temperature being a little "off" the temperature you want to maintain.
Having said that, using something that melts near 21C is a good starting point..
And if you have that, you automatically have the right value of delta S for the material's delta H.
So, as you say what you want is something cheap that "melts" near 21C and has a large delta H per $
I think soft paraffin wax is likely to be a good candidate.
The other thing you need to consider is thermal conductivity. Wax isn't so good at that.
Post by: Eternal Student on 01/06/2022 02:37:00
@chiralSPO - this is what I had started writing but stopped. It's rough, not spell-checked and just not finished. However, if it helps you, then you can see it. You'll realise why I stopped.... it's too long and I started by explaining things too simply, as if other users might also be reading it.
Actually it just is too long.... the forum won't allow me to post anything with too many characters..... I'll split it into two chunks. I'll also put it under a "spoiler" so it won't clog up all the space for people who are reading this thread.
- - - - - - - - - - - -
Chunk 1
Spoiler: show
---End of Chunk 1 ---
Post by: Eternal Student on 01/06/2022 02:39:40
Spoiler: show
Best Wishes.
Post by: chiralSPO on 01/06/2022 12:30:01
Post by: Bored chemist on 01/06/2022 12:40:47
https://en.wikipedia.org/wiki/Trouton%27s_rule
Post by: Eternal Student on 04/06/2022 12:09:23
Come on then @chiralSPO let's have an update on how you're doing.
[Z]T/[A]T = e–RT/(ΔΗ–TΔS)Presumably you noticed that you had the exponent upside down. e-(ΔH-TΔS)/RT should have been there.
However, overall the approach using the equilibrium constant, K, is what we both went for.
I think I kept mentioning that ΔH should be as large as possible BUT it's actually a bit indirect.... it's the size of ΔS that makes the more obvious difference however you need ΔH to be increasing with it just to keep the maximum response of the chemical equilibrium in the right sort of position (at the right temperature).
Anyway... by now I was thinking you might have looked at the last formula, I think it was labelled [Eqn 15] and decided if I made some errors along the way to getting there. If it's alright, you can continue with it, that's what I would have done. That's a thing you can examine directly and optimise it by varying the ΔH and ΔS parameters of the reaction.
Anyway, I don't think you need your two temperatures T1 and T2 just a single figure T where you hope to keep the temperature of the room.
You can develop the idea and see what happens with more complicated reactions involving multiple particle species and having an equilibrium constant involving products of concentrations raised to some powers. I'm not saying I want to do it.... the algebra is only going to get more messy... just that it could be done.
Best Wishes.
Post by: Bored chemist on 04/06/2022 16:04:02
I think I kept mentioning that ΔH should be as large as possible BUT it's actually a bit indirect.... it's the size of ΔS that makes the more obvious differenceYou are talking as if they are independent properties, but once you decide on the temperature you want, there's not much choice.
At equilibrium delta G is zero,
so T Delta S = delta H
What you want to do is store heat and that's delta H so you want that to be big (for a given mass, volume, price or whatever).
Post by: Eternal Student on 04/06/2022 18:02:01
You are talking as if they are independent properties, but once you decide on the temperature you want, there's not much choice.I completely agree. That was the essence of it.
There is one, if quite silly, choice you can make that does allow you to have ΔS and ΔH values chosen independently. You can develop the chemical equilibrium system to have ΔG of the reaction = 0 at some other temperature (not the desired room temperature) and just accept the consequences. You'd have the equilibrium constant k >> 1 or else k <<1 but never the less, it would be some equilibrium with 0 < k < ∞. By Le Chatelier's principle alone if you want to keep it simple, the system will oppose a change in temperature. It can't perform as well as when the system had k≈1 but it will function to some extent. So you can compensate by just making the system massive, i.e. have many molecules of the stuff in the reaction.
To phrase this another way, a massive but badly set-up equilibrium system can still oppose temperature changes and help to hold a room at a given temperature. If it is massive enough then it will be better than a smaller system set up to have ΔG ≈ 0 right at approx. room temperature.
Other than cost of production, the massive but badly set up system may have other undesirable properties: It may not help the person in the room to establish the preferred room temperature as much as the finely tuned and well set up system would. It just maintains it once you have got the room to the preferred temperature initially. Specifically, the system becomes less responsive on one side of the preferred temperature than on the other side of that temperature. Let's say it was set up with ΔH and ΔS values to be an optimal temperature buffer at 0 deg. C then it's effectiveness falls off as you move further away from 0 deg C. So that the person can push the room temperature above the preferred temperature by accident just by leaving their heating system on for an extra half-hour. Meanwhile, if the equilibrium system is set up to have maximum temperature bufferring ability precisely at 22 deg C then it's ability to absorb / release additional heat remains more consistent on both sides of the preferred temperature. Therefore it's more forgiving of a careless person who left the heating on for an extra half hour by mistake - they won't raise the room temperature that much higher than the preferred value.
Best Wishes.