Post by: Tomassci on 26/11/2021 08:59:05
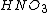 as an important acid in manufacture and agriculture. However, when it comes to phosphoric acid,
as an important acid in manufacture and agriculture. However, when it comes to phosphoric acid,  just straight-up refuses to exist, even though phosphorus is right under nitrogen in the periodic table.
just straight-up refuses to exist, even though phosphorus is right under nitrogen in the periodic table.Why does phosphorus behave in its own way? Why can't it be normal?
Post by: alancalverd on 26/11/2021 11:12:03
tells you how to make it and what it is used for. Interestingly it causes skin burns and eye damage, and its principal use is in cosmetics!
Post by: Bored chemist on 26/11/2021 12:14:23
So the question is why doesn't nitrogen usually follow the rules?
And the answer is largely that it's small.
Though Na3NO4 etc do exist
https://en.wikipedia.org/wiki/Orthonitrate
So it seems they all follow both sets of rules.
Post by: chiralSPO on 29/11/2021 21:17:22
H2O + HXO3
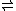 H3XO4
H3XO4There are two reasons that the equilibrium favors the left-hand side when X = N, but the right-hand side when X = P, As, (or Sb, I believe):
1) (the simple answer, pointed out by Bored chemist) nitrogen is the smallest of the bunch (covalent radius of N = 75 pm, P = 106 pm, and As = 119 pm), so it is simply harder to fit the extra oxygen on there.
2) (the complex answer) the 2p orbitals on nitrogen and oxygen have fairly similar energies** (–13.1 and –15.9 eV vs vacuum, respectively) as well as very similar sizes (as noted in point 1), as well as shorter σ bonds (also due to point 1). This means that the p orbitals on neighboring O and N atoms can form very strong π interactions, and that the electrons in the π bonding and π anti-bonding orbitals would both be shared fairly equally.
In contrast, the valence of the phosphorus atom is a 3p orbital, with a much higher energy** (–10.2 eV; again comparing to –15.9 eV for O), and extends much farther away from the nucleus (but not towards a neighboring atom).
This means that in HNO3 the nitrogen atom has much more electron density compared to the P atom in HPO3 (the N is less electrophilic than the P, making the N less likely to accept electron density from another O), while the oxygen atoms bound to N have less electron density than those bound to P (the P-bound O atoms are more basic than N-bound, making them more likely to gain H+) and much stronger N=O bonds than P=O bonds (so it is easier to break the double bonds).
* https://www.schoolmykids.com/learn/interactive-periodic-table/covalent-radius-of-all-the-elements
** https://www.colby.edu/chemistry/PChem/notes/AOIE.pdf