Post by: alkahestcontainer on 31/05/2019 09:39:39
Post by: chiralSPO on 31/05/2019 15:10:50
Trying to precipitate out MgCl2 selectively from this solution would be difficult, but not impossible (though yield and purity will be traded off--ie high purity probably only possible with very small yield, and high yield only possible with low purity).
With a sufficiently high ratio of chloride to sulfate, the solution could be cooled to selectively precipitate out MgCl2. The ratios needed can be derived from looking at their respective Ksp values:
MgCl2(s)
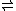 Mg2+ + 2 Cl– Ksp = 738 M3
Mg2+ + 2 Cl– Ksp = 738 M3MgSO4(s)
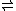 Mg2+ + SO42– Ksp = 4.7 M2
Mg2+ + SO42– Ksp = 4.7 M2So if a starting solution has a 1:1 molar ratio of Mg2+ SO42–, one can calculate just how much chloride would need to be added to favor precipitation of the chloride salt over the sulfate salt.
Based on the Ksp, saturated MgSO4 is about 2.1 M. Plug that in to the MgCl2 Ksp expression, and you will find that the concentration of chloride needed is about 18.7 M. The solubility of HCl in water maxes out at about 12 M, so you would need to cool and/or pressurize the system to accomplish this (I don't recommend actually trying this!)
Alternatively, you could add 1 equivalent of CaCl2 to the solution, which will precipitate out as CaSO4, which can be filtered off, and then evaporation of the water from the solution will leave MgCl2.
I hope this isn't homework...
Post by: chris on 01/06/2019 15:05:59
Post by: Colin2B on 01/06/2019 19:41:31
I shall file away that way of putting it to help explain it to my own kids when they inevitably wonder about this in the future...Me too, can’t wait for them to raise it.
Maybe if they don’t I could subtly introduce it into the conversation 8)
PS I also vote that an excellent explanation @chiralSPO
Post by: Bored chemist on 01/06/2019 20:51:42
In aqueous solution, there is no difference between a solution containing magnesium chloride and sulfuric acid, and a solution containing magnesium sulfate and hydrochloric acid, each just contains Mg2+ ions, H+ ions, Cl– ions, and a mix of SO42– and HSO4– ions (depending on pH).Well...
Trying to precipitate out MgCl2 selectively from this solution would be difficult, but not impossible (though yield and purity will be traded off--ie high purity probably only possible with very small yield, and high yield only possible with low purity).
With a sufficiently high ratio of chloride to sulfate, the solution could be cooled to selectively precipitate out MgCl2. The ratios needed can be derived from looking at their respective Ksp values:
MgCl2(s)Mg2+ + 2 Cl– Ksp = 738 M3
MgSO4(s)Mg2+ + SO42– Ksp = 4.7 M2
So if a starting solution has a 1:1 molar ratio of Mg2+ SO42–, one can calculate just how much chloride would need to be added to favor precipitation of the chloride salt over the sulfate salt.
Based on the Ksp, saturated MgSO4 is about 2.1 M. Plug that in to the MgCl2 Ksp expression, and you will find that the concentration of chloride needed is about 18.7 M. The solubility of HCl in water maxes out at about 12 M, so you would need to cool and/or pressurize the system to accomplish this (I don't recommend actually trying this!)
Alternatively, you could add 1 equivalent of CaCl2 to the solution, which will precipitate out as CaSO4, which can be filtered off, and then evaporation of the water from the solution will leave MgCl2.
I hope this isn't homework...
The SO4 -- ion is roughly as basic as the Cl- ion so, if there's enough HCl to form MgCl2 , there's enough to start converting SO4-- to HSO4-.
And the solubility data you have is for water- rather than H2SO4 / HCl soln- so the calculations will be out.
Post by: chiralSPO on 01/06/2019 21:44:41
Well...
The SO4 -- ion is roughly as basic as the Cl- ion so, if there's enough HCl to form MgCl2 , there's enough to start converting SO4-- to HSO4-.
And the solubility data you have is for water- rather than H2SO4 / HCl soln- so the calculations will be out.
Well, yes, the calculations I provided were a first-order solution (no pun intended). I would be surprised if the sulfate ions change much though... Whatever the protonation state, there can't be more than a 2 M concentration of sulfate/hydrogensulfate/sulfuric acid, while the water is still 55 M. Some of the properties of the solution will change slightly (like melting point, which will be decreased by a few degrees)
I am not sure sure whether the second order considerations would increase or decrease the solubility of magnesium chloride... on the one hand, sulfate ions are better hydrogen bond acceptors than chloride, and the two might compete for hydrogen bonding with the water, which would decrease the solubility of chlorides (increasing the pressure needed to keep the HCl in solution, but also decreasing the solubility of the magnesium chloride salt...). Though, again, there is only a 2 M concentration of sulfate, and 55 M of water, so the sulfate can only tie up so much of the water...
On the other hand, since the sulfate/hydrogen sulfate/sulfuric acid solution is similarly polar to water, and actually contains ions, it should have a higher dielectric constant than pure water (actually, the high concentration of HCl, being roughly an order of magnitude more concentrated, should have more effect than the sulfate...). Higher dielectric constant should increase the solubility of the salt, but obviously, since the solubilities of salts don't increase with increasing concentrations of themselves, this effect is likely not dominant...
Post by: chiralSPO on 01/06/2019 21:48:41
Post by: Bored chemist on 01/06/2019 22:29:50
Some of the properties of the solution will change slightly (like melting point, which will be decreased by a few degrees)The boiling point will drop below room temperature.
Post by: chiralSPO on 01/06/2019 22:45:26
Post by: chris on 03/06/2019 02:22:17
Post by: alancalverd on 03/06/2019 08:07:27
The idea of lowering the bp of water below room temperature may have some astounding applications in space heating or transport, but doing it by stirring epsom salt into stomach acid is surely too simple to have escaped the notice of alchemists and heating engineers?
Anyway what I remember from elementary inorganic analysis (the only fun bit of chemistry!) was that chlorides are more soluble than sulfates, so you'd have to work very hard to extract MgCl2 from the soup.
Or am I getting senile?
Post by: chiralSPO on 03/06/2019 16:06:49
Ummm....when I was a lad, freezing points were depressed and boiling points were elevated by the presence of a solute.In general, that is true--but only when the solute has no vapor pressure. I proposed the use of a nearly 20 M solution of HCl in water, which would definitely have a vapor pressure well above 1 atm at 20 °C (mostly HCl, but some contribution from the water too). So in this case, both the freezing and boiling points are depressed. (which, again, is why I immediately invoked high-pressure systems in the same breath as proposing such high concentrations of HCl).
The idea of lowering the bp of water below room temperature may have some astounding applications in space heating or transport, but doing it by stirring epsom salt into stomach acid is surely too simple to have escaped the notice of alchemists and heating engineers?
Because the vapor is mostly the HCl, it is unlikely to be of much use to the engineers, due it its significant corrosiveness and reduced heat capacity (compared to water), but at least it's not flammable!
Anyway what I remember from elementary inorganic analysis (the only fun bit of chemistry!) was that chlorides are more soluble than sulfates, so you'd have to work very hard to extract MgCl2 from the soup.Well... I can't comment on that last question ;-)
Or am I getting senile?
But I can say that your memory serves you quite well in this instance. The chloride is significantly more soluble than the sulfate, and would require significant effort (ie treatment with several atmospheres of HCl) to crystallize it out selectively.
Or, again, there is the easy way--treatment of the solution with another chloride salt that is freely soluble, but for which the sulfate has very low solubility (I had proposed calcium chloride, for which the Ksp is 1210 M3, and the corresponding calcium sulfate, which has a Ksp of 2x10–5 M2)
Post by: Bored chemist on 03/06/2019 18:43:33
https://en.wikipedia.org/wiki/Sodium_sulfate#/media/File:Na2SO4_solubility.png
it's hard to make reliable generalisations about X are more soluble than Y.
I think silver sulphate is also more soluble than the chloride
Post by: Bored chemist on 03/06/2019 18:44:37
you'd have to work very hard to extract MgCl2 from the soup.High pressure HCl gas probably counts as having to "work very hard".
Post by: alancalverd on 03/06/2019 19:40:25
I think silver sulphate is also more soluble than the chlorideLord! How it all comes back! Chlorides - all soluble except silver. Nitrates - all soluble. Manganese - all salts pink..... And the smell!
Post by: Bored chemist on 03/06/2019 20:04:40
The nitrate of 1,4-Diphenyl-endoanilino-dihydrotriazole is (I think) less soluble than barium sulphate
Acetates (apart from silver acetate) are all soluble.
Nope, chromium (II) acetate is insoluble
Rules are made to be broken.
Post by: evan_au on 03/06/2019 22:51:58
Quote from: OP
Would a mixture of magnesium sulphate and hydrochloric acid create magnesium chloride and sulphuric acid, or would the two simply not react?I think the answer is: Go to your local chemistry supplier (where you bought your magnesium sulphate and hydrochloric acid) and instead buy magnesium chloride and sulphuric acid.
It's likely to be much better for your health (and those around you...)
Post by: Shekhar shah on 21/12/2019 05:11:14
Source:-[COMMERCIAL LINK REMOVED]