Post by: jccc on 25/07/2014 03:26:53
What's the maximum voltage we can create in lab? Thanks
Post by: PmbPhy on 25/07/2014 03:52:39
We know the charges and radius, can we calculate the voltage?Why do you keep ignoring everything that I've explained to you about quantum physics? I told you time and again that particles don't have a position such that it can be said to be at a particular location or radius?
What's the maximum voltage we can create in lab? Thanks
Post by: jccc on 25/07/2014 04:39:21
I don't understand QM, not I don't believe you. Particle don't have a position, how come atom has volume?
Post by: PmbPhy on 25/07/2014 04:45:18
Pete,I kept showing you orbital diagrams by giving you links to them. Didn't you even look at them? If not then see
I don't understand QM, not I don't believe you. Particle don't have a position, how come atom has volume?
http://winter.group.shef.ac.uk/orbitron/
On the left you can click on the various orbitals. They are described by such things as 1s, 2s, 3s, 3p etc. Click on them and you'll see how the electron probability distribution is given. In some instances you can think of that as the "shape" of the atom such as this one
http://winter.group.shef.ac.uk/orbitron/AOs/2p/index.html
Molecules form when the orbitals overlap. That means their sharing the outer electrons in the atom. For that reason they're called "covalent bonds." See http://en.wikipedia.org/wiki/Covalent_bond
The older quantum theory is what you've been thinking about. In the older theory the Bohr model of, say, the hydrogen atom an electron circles the electron only at certain radii but not radiating while orbiting. Only when the electron moves from one orbit to another one with a change of energy does it radiate. The value of the energy radiated is equal to the change in energy levels of the atom and the form or radiation is a photon. See http://en.wikipedia.org/wiki/Bohr_model
The change in voltage is accompanied by a change V in electrostatic energy, eV. An electron in the lowest energy level has a total energy of -13.6 eV.
Post by: jccc on 25/07/2014 05:46:26
Back in school, I was pretty good at those P S N orbitals, I believed everything I learned. One time I even seen in my mind that light wave move like 90 degree double sin wavies.
Getting older, think again, read again, tell you truth, those orbitals on wiki look like fine art.
I am sorry my dear friend, you spent lot of time trying to help me, I still let you down.
What kind of fish should I eat? How many?
Post by: PmbPhy on 25/07/2014 06:33:53
Quote from: jccc
Pete,Was that highschool or college?
Back in school, ..
Quote from: jccc
I was pretty good at those P S N orbitals, I believed everything I learned. One time I even seen in my mind that light wave move like 90 degree double sin wavies.No worries by dear friend. It's the effort that counts. There were things I myself didn't get. I recall one time when I was reading my first text on GR that I came to a paragraph that I just couldn't get. No matter how many times I read it I couldn't understand it. After reading it 50 times do you think I gave up? No. How about after 95 times? Do you think I gave up then? No. What about 99 times? Nope. What about after reading that same paragraph for 100 times! Yes! It was only after reading it 100 times that I finally got it (of course I didn't really count the number of times but it seemed like I read it countless times. I simply got lost as to how many times I read it).
Getting older, think again, read again, tell you truth, those orbitals on wiki look like fine art.
I am sorry my dear friend, you spent lot of time trying to help me, I still let you down.
What kind of fish should I eat? How many?
Just keep trying and understand this - If you're confused about it then you probably understand it better than you think. It's know for being hard to understand because we're simply not used to thinking in those terms. We were born that way. Our brains were wired through evolution to think in terms of classical mechanics, not quantum mechanics. Simply think about this - In quantum mechanics you only know what you can measure. Schrodinger's equation gives you all the information about a system that can be known about the system. For a one particle system, the square magnitude of the wave function
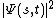 times the volume element of a small region of space
times the volume element of a small region of space 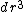 gives you the probability P(r) of measuring the particle to be in that volume centered at the position r. I.e.
gives you the probability P(r) of measuring the particle to be in that volume centered at the position r. I.e.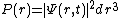
That's the kind of think that quantum mechanics can tell you.
Please don't get me wrong though. Those older models are useful sometimes. In fact sometimes you can imagine the electron moving in circular orbits and you can get a good idea of how magnetism works like that.
Post by: UltimateTheory on 25/07/2014 07:44:26
An electron in the lowest energy level has a total energy of -13.6 eV.
13.6 eV energy is needed to ionize just Hydrogen (proton + electron pair).
In heavier elements like Helium He1+ it's ~54.4 eV, Lithium Li2+ it's ~122.4 eV, and so on. For Gold Au78+ it's ~85 keV.
Ionization energy when there is Z protons in nucleus, and just 1 electron is approximately 13.6 * Z^2.
Post by: PmbPhy on 25/07/2014 10:00:06
Quote from: UltimateTheory
13.6 eV energy is needed to ionize just Hydrogen (proton + electron pair).The subject of this thread is Is there any voltage in between electron and proton in hydrogen atoms?
Post by: jccc on 25/07/2014 10:25:23
An electron in the lowest energy level has a total energy of -13.6 eV.
13.6 eV energy is needed to ionize just Hydrogen (proton + electron pair).
In heavier elements like Helium He1+ it's ~54.4 eV, Lithium Li2+ it's ~122.4 eV, and so on. For Gold Au78+ it's ~85 keV.
Ionization energy when there is Z protons in nucleus, and just 1 electron is approximately 13.6 * Z^2.
Interesting. Make some sense. The more charge in nucleus the stronger attraction to electron therefore need more energy to move it away. Don't get the Z^2 part. Why not 13.6*Z instead?
Post by: UltimateTheory on 25/07/2014 10:32:03
Interesting. Make some sense. The more charge in nucleus the stronger attraction to electron therefore need more energy to move it away. Don't get the Z^2 part. Why not 13.6*Z instead?
All these data are coming from experiments.
Scientists analyze data, and trying to figure out why they have such value not other, trying to create theories explaining results from experiments.
If you analyze data
http://en.wikipedia.org/wiki/Ionization_energies_of_the_elements_%28data_page%29
relation Z^2 is clearly visible.
In exotic atoms such as muonium ionization energy will be completely different.
Post by: UltimateTheory on 25/07/2014 10:34:06
The subject of this thread is Is there any voltage in between electron and proton in hydrogen atoms?
See last post jccc - he didn't know about it. So it was worth mentioning. The whole point of discussion is to learn people things they didn't know before.
Post by: jccc on 25/07/2014 18:38:40
I had enough trying to just learn hydrogen, why you put out heavy atoms at me?
He is Pete, a nice science guy and a brother of ours, please don't mess with him.
All the respect.
Joe
Post by: jccc on 25/07/2014 23:24:57
If that is correct, should any discharge happen?
Post by: PmbPhy on 26/07/2014 04:05:10
I don't know how to convert static force into voltage, but proton and electron at atom radius distance should produce very high voltage, maybe millions ev.Would you like to try the calculation yourself or do you want me to do it for you? The electrostatic potential due to a point charge Q which is located at the origin is given by
If that is correct, should any discharge happen?
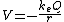
where
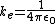 is Coulomb's constant and has the value
is Coulomb's constant and has the value 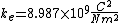 . r is the lowest value in the Bohr model of the atom and has the value
. r is the lowest value in the Bohr model of the atom and has the value 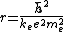 where e = charge of the electron and me is the proper mass of the electron.
where e = charge of the electron and me is the proper mass of the electron.It's a bit of juggling of constants that I'd rather not bother with. Time for you to do some work my friend. :)
Post by: jccc on 26/07/2014 04:25:40
I don't know how to convert static force into voltage, but proton and electron at atom radius distance should produce very high voltage, maybe millions ev.Would you like to try the calculation yourself or do you want me to do it for you? The electrostatic potential due to a point charge Q which is located at the origin is given by
If that is correct, should any discharge happen?
whereis Coulomb's constant and has the value
. r is the lowest value in the Bohr model of the atom and has the value
where e = charge of the electron and me is the proper mass of the electron.
It's a bit of juggling of constants that I'd rather not bother with. Time for you to do some work my friend. :)
Pete, even I calculated the force correct, how to convert it into voltage?
If we connect car battery to two metal plates distance 1 meter, the voltage in between is 12 V, now we move the plates distance to 2 meter, how many V it will be?
When we move more electrons from one plate to another, is the voltage between them increase?
So maybe move 10% electrons produce 10,000 V, 30% produce 1,000,000 v something like that?
Post by: PmbPhy on 26/07/2014 05:52:39
Quote from: jccc
Pete, even I calculated the force correct, how to convert it into voltage?I just told you how to do that. The value that you're looking for is the V in the first equation I gave. Just do the arithmetic out.
Quote from: jccc
If we connect car battery to two metal plates distance 1 meter, the voltage in between is 12 V, now we move the plates distance to 2 meter, how many V it will be?V is independent of how far apart the plates are. All that will change is the strength of the electric field in-between the plates.
Quote from: jccc
When we move more electrons from one plate to another, is the voltage between them increase?Yes.
Quote from: jccc
So maybe move 10% electrons produce 10,000 V, 30% produce 1,000,000 v something like that?The relationship between charge and potential is given by Q = CV where C is the capacitance of the geometry of the conductors.
Post by: jccc on 28/07/2014 02:52:59
If I calculated it right, the voltage in hydrogen atom is about 10^30 volts.
Is that a fact? If so can we make some kind of atom battery?
Post by: PmbPhy on 28/07/2014 05:59:15
A simple little question might move the foundation of the theory of atomic structure.You're confusing a voltage with an EMF. Just because there is a difference in potential somewhere it doesn't mean that it acts like a battery.
If I calculated it right, the voltage in hydrogen atom is about 10^30 volts.
Is that a fact? If so can we make some kind of atom battery?
Post by: evan_au on 28/07/2014 13:37:43
Quote from: jccc
can we make some kind of atom battery?To make a battery (or electrical cell), you need to separate the charges so that one terminal has an excess of electrons, while the other terminal has a deficit of electrons.
The negative electrons can do useful work as they pass through a circuit to get "closer" to the positive charge.
However, an electron in an atom at room temperature is already pretty much as close at it can get to the positive nucleus in that atom; it is forbidden from getting any closer by the laws of quantum mechanics.
To make a battery, you must add a different atom which has a greater attraction for an electron than the atom it is stealing the electron from, as described in this thread: http://www.thenakedscientists.com/forum/index.php?topic=51992.0
Post by: jccc on 28/07/2014 14:23:52
Quote from: jccccan we make some kind of atom battery?To make a battery (or electrical cell), you need to separate the charges so that one terminal has an excess of electrons, while the other terminal has a deficit of electrons.
The negative electrons can do useful work as they pass through a circuit to get "closer" to the positive charge.
However, an electron in an atom at room temperature is already pretty much as close at it can get to the positive nucleus in that atom; it is forbidden from getting any closer by the laws of quantum mechanics.
To make a battery, you must add a different atom which has a greater attraction for an electron than the atom it is stealing the electron from, as described in this thread: http://www.thenakedscientists.com/forum/index.php?topic=51992.0
Quantum law? Well, put a quantum wire cross electron and proton so complete the circle.
Did you mean quantum repulsion = quantum law?
Post by: chiralSPO on 28/07/2014 15:15:38
Quantum law? Well, put a quantum wire cross electron and proton so complete the circle.
What exactly do you think would be the charge carrier in that wire? Macroscopic wires work by transporting electrons. If you somehow had some sort of "quantum wire" connecting an electron to a proton, you still would not get any transfer of charge in either direction because the wire is smaller than an electron. Also the electron and proton don't have any way to donate their charge--their charges are constant and part of what define them as what they are. In the case of an electron the charge is indivisible. I suppose one could argue that the proton's charge could be divided into components based on quarks, but don't expect the positively charged quarks to start leaking out of a proton just because there is a "wire" connecting it to an electron.
Post by: PmbPhy on 28/07/2014 16:15:03
Quote from: evan_au
However, an electron in an atom at room temperature is already pretty much as close at it can get to the positive nucleus in that atom; it is forbidden from getting any closer by the laws of quantum mechanics.That's not quite right. If one uses deep inelastic scattering one can actually probe the inside of hadrons! It was used to confirm the reality of quarks since it showed that protons have substructure. The evidence with protons shows that its composed of three lumps of charge. Cool, huh? :)
See - http://en.wikipedia.org/wiki/Deep_inelastic_scattering
Post by: PmbPhy on 28/07/2014 16:16:48
Quote from: jccc
Quantum law? Well, put a quantum wire cross electron and proton so complete the circle.Why are you referring to "quantum law"? I didn't see anybody mention that term in this thread. And he's right about such wires. It's a meaningless idea.
Did you mean quantum repulsion = quantum law?
Post by: jccc on 28/07/2014 17:47:46
It is forbidden from getting any closer by the laws of quantum mechanics?
Look into the laws, it says there is potential well, energy level etc therefore bla bla so as is. I really cannot get the logic.
Sorry being emotional some times. Hope you enjoy a great day.
Post by: PmbPhy on 28/07/2014 22:34:03
Quote from: jccc
Particle decay may caused by electricity leakage whinin atom. Obviously atom is build by electrical potential.Nope. But what are you talking about specifically? What particles inside the atom are you referring to when you say that they are decaying? Nuclei? Proton? Neutron? Electron?
Quote from: jccc
Seems to complete the puzzle of atom structure, we need a negative sub-charged new particle,Nope. There are currently no missing pieces of any puzzle dealing with atomic structure.
Quote from: jccc
it fills the gape in between nucleus and electrons in atoms, and it fills the whole space to conduct EM wave.That's meaningless.
Post by: jccc on 28/07/2014 23:18:23
Quote from: jcccParticle decay may caused by electricity leakage whinin atom. Obviously atom is build by electrical potential.Nope. But what are you talking about specifically? What particles inside the atom are you referring to when you say that they are decaying? Nuclei? Proton? Neutron? Electron?Quote from: jcccSeems to complete the puzzle of atom structure, we need a negative sub-charged new particle,Nope. There are currently no missing pieces of any puzzle dealing with atomic structure.Quote from: jcccit fills the gape in between nucleus and electrons in atoms, and it fills the whole space to conduct EM wave.That's meaningless.
Pete, maybe I am blind into my soul.
Post by: PmbPhy on 28/07/2014 23:23:56
Quote from: jccc
Pete, maybe I am blind into my soul.That's only because you don't want to give up the idea that nature should be intuitively meaningful. There's no reason to expect that. Our minds were constructed by millions to billions of years of evolution to survive I a macroscopic world. Our minds were never meant to understand the quantum mechanical world. Those of use who accept that easily learn the subject and move on. Those who refuse to accept that fact never understand it and stay in a rut and keep quoting Feynman who said that nobody understands quantum physics. While in a certain sense that has a bit of truth to it, it's often used as an excuse not to learn it.
Post by: jccc on 29/07/2014 00:03:09
Do you understand/agree with the above?
Post by: PmbPhy on 29/07/2014 01:06:30
Quote from: jccc
Whenever a neutron decays, the proton must attract some negative goo from somewhere, ...What is "goo"? By the way. Neutrons don't decay while they're in the nucleus.
Quote from: jccc
... so we are looking for a continuum of negatively charged....particles?First you started out with one neutron decaying into one proton which attracts one negatively charged particle such as an electron. Why the continuum of negatively charged particles? And negatively charged particles cannot form a continuum because they're quantized, i.e. come in discrete amounts.
Quote from: jccc
Do you understand/agree with the above?As usual, you're not making any sense.
Post by: jccc on 29/07/2014 05:21:40
So far, it seems that charge is quantised, but if you can find and demonstrate a continuous subquantum of charge, you may have a point.
Pete, that's from a comment about my atomic structure theory. Read his other comments learned he knows lot more than me. Whole lot more.
Post by: jccc on 30/07/2014 15:13:06
If high voltage within atom is real thing, why can't we utilize it?
It is like a water reserver in the air, all we need is to open it to release the water.
Post by: UltimateTheory on 30/07/2014 16:52:36
If high voltage within atom is real thing, why can't we utilize it?
There is no high voltage within atom..
It is like a water reserver in the air, all we need is to open it to release the water.
I told you how to do it - using antimatter/antiparticles.
That's the only way to get the all energy from rest-mass of f.e. Hydrogen.
Post by: JP on 30/07/2014 17:45:58
-The Mods
Post by: PmbPhy on 30/07/2014 23:58:54
Quote from: UltimateTheory
I told you how to do it - using antimatter/antiparticles.Note: I believe that when UT wrote get the all energy he reallly meant to write get all the energy. Therefore when I quote him below I'll use the later phrasing.
That's the only way to get the all energy from rest-mass of f.e. Hydrogen.
Using matter/antimatter annihilation to get all the energy from rest mass is a very common misconception. This is all explained in the article Does nature convert mass into energy? by Ralph Baierlein, Am. J. Phys., 75(4), Apr. (2007). See - http://scitation.aip.org/content/aapt/journal/ajp/75/4/10.1119/1.2431183
I put the article on my website at http://home.comcast.net/~peter.m.brown/ref/baierlein.pdf
The abstract reads
Quote
First I provide some history of how the equation E=mc2 arose, establish what “mass” means in the context of this relation, and present some aspects of how the relation can be understood. Then I address the question, Does E=mc2 mean that one can “convert mass into energy” and vice versa?
In it the author writes
Quote
Q. Does the equation E = mc2 mean that one can "convert mass into energy" and vice versa"?
A. Not really, but the issue is complex, and eminent physicists have used that phrase and variants of it.
This is only one article that points this out. Any physics journal article that I've ever read on the subject agrees with the conclusion of this article. And I've read a lot of articles on this subject. Regardless of what I read I know what the author says is true even without reading it. Proof given upon request. The main part of the argument is that energy is conserved so that the amount of energy that you start with is the same energy you end up with.
When people refer to changing mass to energy using electron/positron annihilation what they really mean is that they start out with rest mass and end up with photons which are all kinetic energy and zero rest mass. The energy of photons is said to be all kinetic energy.
It's also a common misconception to believe that when a proton and antiproton annihilate the end result is all photons/kinetic energy with zero rest mass. That too is not true. In such annihilations you end up with either
 or
or 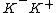 . But to be precise see http://en.wikipedia.org/wiki/Annihilation#Proton-antiproton_annihilation which explains
. But to be precise see http://en.wikipedia.org/wiki/Annihilation#Proton-antiproton_annihilation which explainsQuote
When a proton encounters its antiparticle (and more generally, if any species of baryon encounters any species of antibaryon), the reaction is not as simple as electron-positron annihilation. Unlike an electron, a proton is a composite particle consisting of three "valence quarks" and an indeterminate number of "sea quarks" bound by gluons. Thus, when a proton encounters an antiproton, one of its constituent valence quarks may annihilate with an antiquark, while the remaining quarks and antiquarks will undergo rearrangement into a number of mesons (mostly pions and kaons), which will fly away from the annihilation point. The newly created mesons are unstable, and will decay in a series of reactions that ultimately produce nothing but gamma rays, electrons, positrons, and neutrinos. This type of reaction will occur between any baryon (particle consisting of three quarks) and any antibaryon (consisting of three antiquarks). Antiprotons can and do annihilate with neutrons, and likewise antineutrons can annihilate with protons, as discussed below.
Post by: PmbPhy on 31/07/2014 00:40:48
Quote from: jccc
If high voltage within atom is real thing, why can't we utilize it?Didn't you ever calculate it like I showed you? It's even easier to get an estimate. Suppose
r = 5.3x10-11m
k = 8.99x109Nm2/C2
e = 1.6022x-19
V = ke/r
Then upon plugging these in we get to two significant figures
V = 27 Volts
That's hardly high voltage.
Quote from: jccc
It is like a water reserver in the air, all we need is to open it to release the water.
Post by: jccc on 31/07/2014 00:51:49
Quantum law? Well, put a quantum wire cross electron and proton so complete the circle.
What exactly do you think would be the charge carrier in that wire? Macroscopic wires work by transporting electrons. If you somehow had some sort of "quantum wire" connecting an electron to a proton, you still would not get any transfer of charge in either direction because the wire is smaller than an electron. Also the electron and proton don't have any way to donate their charge--their charges are constant and part of what define them as what they are. In the case of an electron the charge is indivisible. I suppose one could argue that the proton's charge could be divided into components based on quarks, but don't expect the positively charged quarks to start leaking out of a proton just because there is a "wire" connecting it to an electron.
The middle shell electron cloud should be the current carrier without wire. Maybe?
Post by: PmbPhy on 31/07/2014 01:08:39
Quote from: jccc
The middle shell electron cloud should be the current carrier without wire. Maybe?No.
Post by: UltimateTheory on 31/07/2014 01:50:22
It's also a common misconception to believe that when a proton and antiproton annihilate the end result is all photons/kinetic energy with zero rest mass. That too is not true. In such annihilations you end up with eitheror
.
Pair of 2 mesons is very very rare. They happens just in percents of annihilations.
And neither pair of pion-, pion+, nor pair of kaon-,kaon+ are stable. So they quickly (within micro seconds usually), decay.
The most common proton-antiproton annihilation branch is to 3 pions 0, AFAIK.
And the most common pion0 decay branch is to 2 gamma photons. This gives us, the most common (the most probable) branch of decay p+p- ->
 -> 6 gamma photons.
-> 6 gamma photons.Which is what I wrote 2 weeks ago to jccc in thread:
http://www.thenakedscientists.com/forum/index.php?topic=26362.msg438099#msg438099
post #140
Post by: PmbPhy on 31/07/2014 10:52:40
Quote from: UltimateTheory
Pair of 2 mesons is very very rare.And yet if I didn't mention them I'd have risked being remiss.
Quote from: UltimateTheory
And neither pair of pion-, pion+, nor pair of kaon-,kaon+ are stable.Why would you even say such a thing when I gave the reference which explains
Quote
The newly created mesons are unstable, and will decay in a series of reactions that ultimately produce nothing but gamma rays, electrons, positrons, and neutrinos.
Quote from: UltimateTheory
The most common proton-antiproton annihilation branch is to 3 pions 0, AFAIK.
You missed the point. You implied that they all end up as photons when in fact
Quote
The newly created mesons are unstable, and will decay in a series of reactions that ultimately produce nothing but gamma rays, electrons, positrons, and neutrinos.So the series doesn't always end up with photons but with electrons, positrons and neutrinos.
Post by: UltimateTheory on 31/07/2014 12:48:29
So the series doesn't always end up with photons but with electrons, positrons and neutrinos.
True.
But it is in minority of cases.
I don't have to explain the all decay modes every time.
Enough is explaining the main branch, and give reference link for details, for rare exceptions.
(especially explaining something to non-physicist that won't understand quarter of it)
Post #140 in that thread was to jccc. So the same here. No need to repeat writing the same multiple times to the same person.
You should instead concentrate on answering to jccc, as he wants to get 938.272 MeV energy from proton, without any annihilation..
Post by: PmbPhy on 31/07/2014 14:49:23
Quote from: UltimateTheory
You should instead concentrate on answering to jccc, as he wants to get 938.272 MeV energy from proton, without any annihilation..If that's what he indicated or implied that he wanted I would. But that's not what he's looking for. Just ask him and he'll tell you. Right, Joe?
In any case the assertion That's the only way to get the all energy from rest-mass of f.e. Hydrogen is questionable since the energy is already there to begin with. There's no "getting" energy from anywhere. Unless you really meant that its the only way to change the form of energy, i.e. from rest energy to kinetic energy? Is that it?
Post by: PmbPhy on 31/07/2014 14:53:19
Quote from: UltimateTheory
True.Then you should have said "most" rather than "all."
But it is in minority of cases.
Quote from: UltimateTheory
I don't have to explain the all decay modes every time.You should. Never imply something happens all the time if it doesn't. The author of the text I'm proof reading made this same mistake until I pointed it out to him. When he realized that he made that mistake he changed it quickly.
re - Enough is explaining the main branch, and give reference link for details, for rare exceptions. - If that's the case then please show me where you're getting your data from. I want to see this for myself.
Post by: UltimateTheory on 31/07/2014 15:17:29
If that's what he indicated or implied that he wanted I would. But that's not what he's looking for. Just ask him and he'll tell you. Right, Joe?
See post #139 by jccc in thread http://www.thenakedscientists.com/forum/index.php?topic=26362.msg438098#msg438098
Post by: PmbPhy on 31/07/2014 15:33:58
Okay. Although why you took so long to answer is odd. No wonder I missed it.If that's what he indicated or implied that he wanted I would. But that's not what he's looking for. Just ask him and he'll tell you. Right, Joe?
See post #139 by jccc in thread http://www.thenakedscientists.com/forum/index.php?topic=26362.msg438098#msg438098
That doesn't answer my other question, where did you get your data from? I want to confirm that what you claim is true.
Post by: jccc on 31/07/2014 16:36:54
Post by: PmbPhy on 31/07/2014 18:27:59
May I collect a little transpassing fee? Pretty broke lately.What exactly is transpassing?
Post by: jccc on 31/07/2014 18:48:54
May I collect a little transpassing fee? Pretty broke lately.What exactly is transpassing?
Every time I am nearing broke, making lot typos. Never been there Pete?
Post by: jccc on 02/08/2014 06:55:45
Quote from: jcccIf high voltage within atom is real thing, why can't we utilize it?Didn't you ever calculate it like I showed you? It's even easier to get an estimate. Suppose
r = 5.3x10-11m
k = 8.99x109Nm2/C2
e = 1.6022x-19
V = ke/r
Then upon plugging these in we get to two significant figures
V = 27 Volts
That's hardly high voltage.Quote from: jcccIt is like a water reserver in the air, all we need is to open it to release the water.
Thank you Pete, hope you like my Jokes.
V=Ke/r, so V and r dependent? Why is voltage between two points in a conductor independent with distance?
Also 27 volt is a lot compare with a car battery, can we use liquid hydrogen put in two metal plates to make a 27 V battery? Electron move to anode plate, proton move to the cathode plate.
Post by: jccc on 05/08/2014 07:45:32
Isn't he always right?
Post by: evan_au on 05/08/2014 11:25:15
Quote
why can't we use the 27 volts within H atom as a battery?
You must have another participant in the reaction which applies the 13V needed to extract an electron from the Hydrogen 1s shell (13eV of energy).
When the electron falls back into the ground state, it will then release this 13V (sometimes as a 13eV ultraviolet photon).
So it's not very useful to have a battery which consumes 13V to produce 13V. Given inefficiencies of typical chemical reactions, you are better off with a brick.
The reason it does not produce 27V is that the electron can't fall all the way into the nucleus, due to the quantum nature of electrons and atoms.