Post by: Eternal Student on 18/07/2021 18:31:38
Na(s) + H2O (l) → Na(OH) (aq.) +
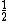 H2 (↑)
H2 (↑)Or something similar happens when solid sodium is carefully put into contact with some liquid water at room temp & pressure.
Now does the sodium atom really need to be in such close proximity to water? The sodium atom tends to form an ion and the reasonably free electron would seem to be able to travel a bit. How far away can the sodium be kept from the water and still have the reaction proceed? Do they need to be within a few Angstroms of each other or are we taking about something like a millimetre or more? (You know my follow up question will be: Accepting that the reaction rate may be small can we have them one full metre apart etc.)
Water vapour in the air might be an issue, let's say we do the experiment in unsatuared air. Perhaps we could put the experiment in a partial vaccum maintained by a pump and keep the liquid water temperature low so that it doesn't tend to vaporise.
Post by: Bored chemist on 18/07/2021 20:28:17
But you could put the sodium near the water in a tube with dry gas- for example nitrogen- flowing through it to keep the water vapour away from the sodium.
For any practical purpose the sodium and the water need to be in contact to react.
Hypothetically the electrons and atoms can tunnel around and react.
With other reactions- in the vapour phase- you can get odd looking results which appear to suggest that the reaction happens before they atoms are in contact,
https://en.wikipedia.org/wiki/Harpoon_reaction
Post by: Eternal Student on 18/07/2021 21:00:27
Post by: Bored chemist on 18/07/2021 21:28:08
It's like measuring the size of a blob of cotton wool. It depends on how hard you squeeze it.
Post by: Eternal Student on 19/07/2021 00:41:51
I've read the link about harpoon reactions. Yes, that makes sense. It seems to be based on a fairly conventional particle theory (Collision theory and conventional reaction rate kinetics). I wouldn't have known to use the term "harpoon reaction", so that information has saved me an hour. Thank you.
I was thinking along the lines of Quantum Mechanics. I appreciate that atoms have no clearly defined radius (this is mainly why I said do the atoms need to be within a few Angstroms).
The only other chemical reaction that I knew a little bit about and involved a significant separation between reactants is rusting. Here the lump of Iron can pass electrons to other Iron atoms without difficulty. An iron pipe needs the presence of water and oxygen (air) to rust but the actual process of rusting is an electrochemical reaction where anodic and cathodic regions of the iron can be separated by some distance. (I probably don't need to tell Bored_Chemist about rusting, so I'll stop here).
Anyway, I've got enough to think about and continue google-ing for this evening. Thanks for your time.
Post by: chiralSPO on 19/07/2021 05:23:15
Let's say this "chunk" of sodium is about 1 cm on a side (1 cm3 of sodium = 970 mg of sodium = 2.55×1022 sodium atoms).
One of the properties of metals (one of the *defining* properties, depending on the context) is that the valence electrons are completely delocalized: the proverbial "sea of mobile electrons." It isn't really meaningful to say that the electron came from any one atom (barring defects or other special cases). Thus when an arbitrarily large chunk of sodium comes into contact with water, one could reasonably interpret electrons moving from being centered within the arbitrarily large piece of sodium, to moving into the σ* orbitals of neighboring water molecules. Depending on how you want to define things, it could be an arbitrarily far electron transfer.
One interesting effect of this is that, if the metal is a liquid, the resulting electrostatic forces resulting from this long-range "harpoon" reaction (thanks Bored, I have never come across that term before either, but it is a great concept!), distort the surface of the liquid metal into tiny spikes, thereby increasing the exposed surface area, and this can result in a "Coulomb explosion"
This is excellently demonstrated in this video (best at 15:45): (note: I am aware that the guy who made this video is associated with some controversy, but this particular minute of this video addresses the question very directly).
Post by: Eternal Student on 19/07/2021 10:07:56