Naked Science Forum
Non Life Sciences => Chemistry => Topic started by: Indranil on 01/12/2017 01:58:27
-
As we know in NH3, N full fills its octet with three single bonds with H and a lone pair of electrons and so H. So this molecule is stable. In BF3, F full fills their octets by three single bonds with B but B is unable to fulfill its octet. It got 6 electrons by three single bonds with F. So, B needs 2 more electrons to fulfill its octet. So, BF3 receives the lone pair of electrons from the NH3 and make a coordinate covalent bond between NH3 and BF3, making a new molecule NH3BF3.
So my question is how to calculate the proton numbers in "NH3+'' after giving the lone pair of electrons and ''BF3-'' after receiving the lone pair of electrons?
-
Using the Lewis structure formalism, the correct formal charges would be +1 on N and –1 on B. Lewis structures are not great descriptions of compounds and ions containing boron (or aluminum). B2H6 is a real head-scratcher from a formal oxidation state point of view, and (Al(CH3)2 even crazier!
Molecular orbital theory is a much better way of thinking about such species.
http://www.chem.ucla.edu/~cantrill/30A_F05/Diborane.pdf

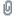 Screen Shot 2017-11-30 at 10.20.47 PM.png (49.5 kB . 698x614 - viewed 18662 times)
Screen Shot 2017-11-30 at 10.20.47 PM.png (49.5 kB . 698x614 - viewed 18662 times)
As you can see, H3N-BH3 is very similar to H3C-CH3
***disclaimer on MO diagram*** I generated this myself using back-of the envelope techniques, and it is late at night here, so do not take the precise orbital energies or ordering literally.
-
and note that only electrons are moving around... no protons are transferred.