This week, a new discovery suggesting that the chemistry of life could have come to Earth in a meteorite, how foetal immunity protects the expectant mother, and why we need to be careful with stem cells - a new study has found that they have an above average chance of carrying cancer-causing genetic changes. Plus, a new technique to etch graphene sheets with single-atom precision.
In this episode

00:23 - Key component of life's building blocks could have come from space
Key component of life's building blocks could have come from space
The origins of life on earth are a hotly debated topic among scientists. One theory suggests that, even if whole organisms didn't come to earth carried by meteorites, then maybe meteorites brought some of the chemical building blocks for amino acids - the molecules that make up proteins. Now tests on a meteorite with the catchy name CR2 Grave Nunataks 95229 provide more evidence that meteorites might have brought these building blocks to earth, kickstarting the chain of events that led to the evolution of life here.
CR2 Grave Nunataks 95229 is a type of meteorite called a carbonaceous chondrite - meteorites that contain a range of organic chemicals including amino acids. Because of this, some scientists think that they might have 'seeded' earth with these chemicals when they fell from space, providing the primitive building blocks for the formation of DNA and proteins, which ultimately led to life as we know it. But studies of similar meteorites haven't come up with solid evidence for this, as the chemicals they contain are a real mix of all kinds of things - most of which couldn't easily be used to create molecules of life.
 The Grave meteorite spun off from an asteroid and landed in Antartctica in 1995. Researchers, led by Sandra Pizzarello and her colleagues in the US, analysed the chemical makeup of the meteorite using high pressure water at a temperature of 300 degrees centigrade - conditions designed to mimic both the asteroid where the meteorite was made, and the conditions on the early earth.
The Grave meteorite spun off from an asteroid and landed in Antartctica in 1995. Researchers, led by Sandra Pizzarello and her colleagues in the US, analysed the chemical makeup of the meteorite using high pressure water at a temperature of 300 degrees centigrade - conditions designed to mimic both the asteroid where the meteorite was made, and the conditions on the early earth.
Publishing their results in the journal PNAS this week, the team discovered that their asteroid contained surprisingly high amounts of ammonia - a chemical precursor to amino acids. These levels were much higher than might be expected on earth at the time.
Given the chemical makeup of similar meteorites, and the fact that most of these only contain compounds built of rings of carbon atoms, this is an unusual find indeed, and the first of its kind. Further analysis showed that the ammonia in the meteorite could only have come from the original asteroid it came from, and suggests that there was a lot of ammonia around in that environment.
Nitrogen, which is a key part of ammonia, is the fourth most common reactive element in the universe. Here on earth, it's a vital component of proteins, as well as DNA and RNA - the genetic information within living cells - and it's indispensible for life. Ammonia plays a key role in many chemical reactions, including the reactions that create the molecules of life.
But from what we know of the conditions on the early earth, it's been hard for scientists to figure out how this might have worked back then. For a start, evidence suggests that the early earth's atmosphere wasn't relatively rich in ammonia. And we know that sunlight can also break down the chemical, which would have been a big problem at the time.
But the discovery that meteorites can actually contain relatively large amounts of ammonia suggests an alternative route for the chemical to turn up and get involved in the chemical reactions that might have led to the generation of the molecular building blocks of life all those millions of years ago. And it adds weight to the idea that at least some of the molecules that kickstarted life on earth may have come from space.

04:03 - Stem cell mutants
Stem cell mutants
Stem cells made by reprogramming a patients own cells have the potential to revolutionise personalised medicine. But are they safe?
A paper in Nature this week urges caution after finding that current techniques involving the addition of up to four genes to "reprogramme" adult cells so that they revert to a stem cell state can also leave the cells harbouring potentially harmful mutations, some capable of causing cancer.
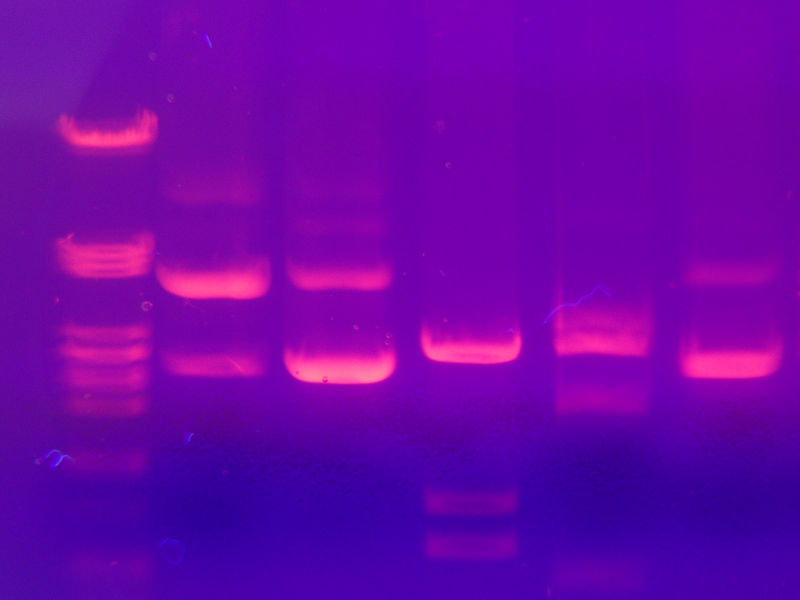 Study author Kun Zhang and his colleagues genetically sequenced 22 lines of so-called iPS cells - induced pluripotential stem cells - produced by seven different laboratories using several different techniques. They then compared the resulting genetic profiles with the corresponding sequences of the parent cells from which the stem cells had been made. They were surprised to find tenfold more mutations (DNA changes) in the stem cells than would be expected normally for cells kept in culture, and these changes appeared to be permanent, remaining present when the cells were grown for over 40 generations.
Study author Kun Zhang and his colleagues genetically sequenced 22 lines of so-called iPS cells - induced pluripotential stem cells - produced by seven different laboratories using several different techniques. They then compared the resulting genetic profiles with the corresponding sequences of the parent cells from which the stem cells had been made. They were surprised to find tenfold more mutations (DNA changes) in the stem cells than would be expected normally for cells kept in culture, and these changes appeared to be permanent, remaining present when the cells were grown for over 40 generations.
They were also not a random smattering of changes but instead were more than twice as likely to be "missense" mutations that would directly alter the structure of the protein encoded by the affected gene rather than silent "synonymous" changes that don't actually alter protein structure.
This indicates that strong selection is at play when the cells are being produced and that those that tend to grow best might be the ones that have more of these sorts of changes.
The researchers aren't sure yet what renders the cells more vulnerable to mutation during the reprogramming process, or when the changes occur.
"These studies look at two different aspects of stem cell mutations," says Zhang, "but their take-home message is the same - things can go wrong at the genome level when reprogramming and growing reprogrammed cells. So, to maximise safety, before we put these cells back in the human body for therapeutic purposes, we must be sure that the cells contain the same genome as the recipient, with no cancer-causing or other serious types of mutations."
07:42 - Graphene Lithography Atomic Precision Etching
Graphene Lithography Atomic Precision Etching
Professor James Tour, Rice University
Kat - Also in the news this week, researchers at Rice university have developed a new way to etch structures into stacked piles of graphene, the marvellous material consisting of a single layer of carbon atoms. This could allow manufacturers to make computer chips from graphene in much the same way they currently do for silicon. But sometimes in science, you make a great discovery entirely by accident - and this was just one of those cases, as Professor Jim Tour explains..
Jim - What we set out to do was to convert graphene to graphein, and what that is, is taking the carbon structure of graphene which is a bunch of 6-member rings in a plane and attaching hydrogen to it. That makes then graphein, and that would make an area that would be non-conductive. We thought if we could pattern zinc upon graphene, we could then use the hydrogen which is generated from the zinc reduction reaction from where zinc is treated with acid to hydrogenate the graphene to graphein. But what happened was, it turned out that wherever the zinc landed, it removed the graphene layer, but left the underlying layer completely intact.
Chris - It's really reminiscent of what people do with silicon and lasers to make microchips, isn't it? But you're doing this using zinc and graphene which is interesting because people are talking about using graphene as a material to make the next generation of microchips.
Jim - Precisely and so, this constitutes lithography. Lithography is the way we make computer chips. You take a big silicon wafer and you chip away at it using chemicals and light to make the small features, namely transistors and wires for example. So one takes a mask and has certain areas as holes from the mask and then shines light through those holes and that will develop what are called "resists" on top of the silicon to develop and build up these structures that we've seen on chips. But now, to be able to do this in graphene brings graphene one step closer. There is a huge difference between a monolayer and a bilayer of graphene. A monolayer of graphene is a metal. It doesn't have a band gap. It's not something that's easily made into a transistor, but if you have two layers of graphene, it opens up a gap and it becomes a little bit more like silicon where you can make it into a transistor. And so, to be able to have one layer or two layers, or three layers which can be more conductive then you can have different devices next to each other, and that's what you want. You want to have heterogeneity in devices. You don't want all devices to be exactly the same. So it's a new tool and a toolbox for making graphene into electronic chips.
Chris - Do you actually know though, Jim what the zinc is doing? Why this actually works to strip away the single layers and leave the one underneath untouched?
Jim - I think we have a reasonable idea. So what happens is the zinc is spattered on the surface so that will cause zinc atoms to fly up from a chunk of zinc metal, and to hit the surface that we're trying to pattern. You make a mask and wherever you want the metal to go, you have holes in the mask and it hits the surface. So what happens is about 0.5% of the zinc atoms, come with enough energy to actually knock out a carbon atom from the graphene and substitute in with the zinc atom. The zinc metal has a very high oxidation potential, so it's really begging to oxidise rather rapidly and that's going to then leave, cause the zinc atom to come out and you'll get oxygen-carbon bonds. So wherever a zinc atom had been, now the carbon atom is knocked out and the surrounding carbon atoms become oxygenated. So what you end up with is, instead of a sheet of graphene now, you have a sheet of graphene with holes in it. Then what's done is we put it into acid and acid then strips away the zinc, and in stripping away the zinc, it generates hydrogen. And that bubbles and helps to wash away the small pieces of graphene that have now been diced up on the surface. But the zinc atoms that hit never had enough energy to go through one layer and affect the second layer below. And so, it turns out to be quite a selective technique that's not only shown now with zinc. We showed we could do it with aluminium as well. So that's a reasonable understanding that we have now of the mechanism.
Chris - And once you cut through by substituting oxygen onto some of these area so they may be removed or floated off, the residual graphene from the layer where you've got - say, a step is the bit that remains behind stable or will that chemically deteriorate with time?
Jim - Everything that we have seen at the step edge, where you have one sheet of graphene that is one step higher than the sheet below it, is stable. We haven't seen that curling up. We haven't seen that undergoing any problem. It undoubtedly has different atoms at the edge. There's going to have to be - they're hydrogen atoms or oxygen atoms at that very edge. But no, there is no delamination that occurs. The other fascinating point about this is lithography is always done in industry but we have hit the ultimate in lithography. It is single atom layer precision. It will never get better than this. So in other words, in a thousand years after doing lithography, they can't do better than this. We are stripping off a single atomic layer. You can't cut an atom in half. That's as thin as you're going to get. This shows that we can have precision that you could never have in silicon - single atom resolution.
Chris - And you think that this will be a practical way to make, if you had to - a microchip of the future using graphene as a base material.
Jim - It's certainly a new wrench in the tool box. There was no way to do this before, so if we are going to make large scale patterns out of graphene, this is definitely a way to do it. And it uses methodologies that are commonly used in silicon manufacture.
Kat - ...which will make ousting silicon from its position at the heart of the microprocessor industry that bit easier. That was Professor Jim Tour, from Rice University - he's published that work in the journal Science this week.
14:23 - Foetal immunity protects females
Foetal immunity protects females
The female genital tract uses a foetal gene to protect itself from infection during adulthood, new research has found.
Mucosal surfaces, like the genital tract, mouth, gut and eyes, are important portals of entry for pathogens attempting to penetrate our immunological defences. They are known to be defended by a class of antibodies known as IgG as well as a number of other chemical armaments, but exactly how the IgG antibodies reach the skin surface where they can neutralise attacking microbes, no one was sure.
 Now, writing in PNAS, University of Maryland researcher Xiaoping Zhu and his colleagues have discovered that adult cells use a gene normally found working in the placenta to do the job. The gene in question is called FcRn and it's role during pregnancy is to grab antibodies from the mother and add them to the baby's bloodstream. This temporarily provides the newborn with so-called "passive protection" from the mother while its own immune system gains momentum after it is born.
Now, writing in PNAS, University of Maryland researcher Xiaoping Zhu and his colleagues have discovered that adult cells use a gene normally found working in the placenta to do the job. The gene in question is called FcRn and it's role during pregnancy is to grab antibodies from the mother and add them to the baby's bloodstream. This temporarily provides the newborn with so-called "passive protection" from the mother while its own immune system gains momentum after it is born.
Reasoning that the same gene might be at work in the genital tract, the Maryland team tested a range of cultured cells include uterine, cervical and vaginal lines, confirming the presence of high levels of FcRn activity.
Next, to confirm that the gene was actually functional, they incubated cultured cells that were expressing FcRn with IgG-class antibodies. Intriguingly the antibodies were picked up and passed from one side of the cell to the other, a process which ceased when the researchers disabled the FcRn gene.
They were also able to confirm the same results in mice, including demonstrating that chemically-labelled antibodies are transported onto the mucosal surfaces of the genital tract by the FcRn gene. When this was genetically "knocked out" from the mice, the antibody movement was arrested. These knockout animals were also highly susceptible to infection with a strain of herpes virus that transmits genitally.
Knowing how this process now works, the researchers argue that it may be possible to exploit this system to produce better vaccines capable of boosting immune defences at mucosal surfaces and hence better protect patients against a range of infections, not least HIV and other sexually-transmitted diseases.
Related Content
- Previous Making Medicine - How Tablets are Made
- Next Aspirin's Anniversary









Comments
Add a comment