Eureka Streaker: Experiments that Changed the World
From Archimedes leaping from his bath shouting Eureka, to Isaac Newton's falling apples and Volta's piles that produced electricity on tap, this week we recreate some of the scientific experiments that changed the way we view the world. Join Ginny Smith and Chris Smith on a journey through two thousand years of discovery that includes bricks on ropes, a singing Solar System, a hydrogen detonation and a spectroscope...
In this episode

00:49 - The Eureka moment: Archimedes' principle
The Eureka moment: Archimedes' principle
with Dr Paul Coxon, Cambridge University
Materials scientist Paul Coxon takes us back to ancient Greece, to explain the basis of one of the most famous experiments of all time and one which made Ancient Greek mathematician, physicist, engineer, inventor, and astronomer Archimedes a household name 2000 years later, as he explained to Chris Smith...
Paul - I'm going to show you Archimedes' principle. It's a very ancient experiment and it uses how materials behave in fluids to solve a historical fraud.
Chris - When you say Archimedes, this is the bloke that ran down the street in the nude, isn't it?
Paul - Eureka! Yes, he put himself in the bath and this is how he came to the discovery.
Chris - So, he was a eureka-streaker.
Paul - He was a eureka-streaker. I'm not naked. I'm going to show you with some plasticine.
Chris - So, we have in front of us two tanks with blue liquid and they have a sort of tap coming out of the side of the tank.
Paul - Yes and the water is just levelled with the tap. So, when we put anything in the water, the level will rise and it will trickle out. What we're going to do is we're going to immerse some materials in the water, raise the level and measure how much water is displaced, how much water is pushed out of the way.
Chris - In front of the tanks, we've got two balances, weighing scales. So, the water will come trickling out of the tank and we'll be able to weigh how much weight of water comes out when you put the objects into the tank.
Paul - Yes and if we measure the weight of the water, because we know the density of the water, we know the volume of water which is being pushed out of the way. This is a very important property of buoyancy. When you put a body in water, it displaces the water out of the way. So, I've got a little boat here and this is how a boat floats. It pushes the water out of the way and the weight of the water, which is being pushed out of the way is equivalent to the weight of the boat pushing downwards. These balance each other out and this is how the boat floats. It's called buoyancy.
Archimedes worked for a king, a tyrant and the Greeks were fighting against the Romans and they had a military victory. So in tribute, the King made a votive wreath to be placed on the statue of a god in a temple. So, the King gave his goldsmither a known mass of gold. He knew how much gold there was and he said, "Go off, melt that, make it into a beautiful wreath for me." But he was very suspicious. He thought that the goldsmith had taken a little bit of gold and had kept some for himself and built up the rest of the gold with some cheaper material like lead or copper.
Chris - Now of course, that would mean that the crown would look the same but it wouldn't be all gold. There'd be something else in there so the weight might not be quite right.
Paul - It would look the same and it would weigh the same. So, if we just check on our balancers now, we can see that I have a mass of gold and I have a crown, and they're both exactly the same, 524 grams. So, they both look the same but they have different volumes.
Chris - So, we've got two plasticine objects, one big chunk of plasticine in a big blog, one which you've made into your crown. They both weigh 524 grams but I don't know if both of them are made of only plasticine.
Paul - That's the thing. That's the challenge which Archimedes was trying to solve. He wanted to know the purity of the crown. But he couldn't change the shape of the crown. He couldn't melt it because it was a sacred object. What he needed to do, he needed to measure the volume. So, what I'm going to do now is put in our known gold samples and it should displace a known volume of water.
Chris - So, we're putting one blob of the plasticine into one of the tanks and water is now trickling out into the bowl. Now, we've got the crown which is - we know it weighs exactly the same but we know it's made of plasticine, perhaps with something else added, and we're now going to put that in the other tank.
Paul - And we're going to displace the water and we're going to measure how much water the crown displaces.
Chris - So basically, because the objects are taking up volume or space inside the tanks, they've raised the level of the water above the level at which the pipe comes out in the water that's being pushed upwards is now flowing out and we're weighing it.
Paul - Yes and this is what Archimedes discovered in the bath. When he was given this problem to solve, he went on to one of his very rare baths in a public bath and as he lowered himself into the water, he noticed the water level rose up and it slopped over. This is what inspired him to do this experiment. We use it today in the design of ships and submarines. So, although it's a very ancient experiment, it has real life applications today.
Chris - So, this is the non-crown plasticine. This is almost finished and we're at 349, 350 grams of water. So, that's about 350 millilitres of water, isn't it? So, the volume that we've put in must be about 350 millilitres. So, the crown one, we've now got a total mass of water of 370 grams has come off. So, the crown is actually displacing more water than the original blob of plasticines. What does that tell us?
Paul - This tells us that the volume of the crown, although it weighs the same as this gold, the gold standard, its volume is bigger so its density is less. So, this was a key way of finding out that the density had been changed, had been cut and mixed with some cheaper material. Unfortunately, the poor goldsmith, he lost his head.
Chris - And you've made your one less dense because it's got some balls of wood in there - by the look of it - your crown.
Paul - Yes. So, wood is less dense. So, it bulks up the volume and it displaces more water.
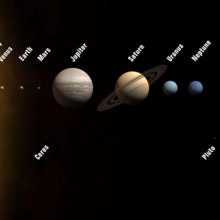
06:42 - The harmony of the planets
The harmony of the planets
with Prof Gerry Gilmore, University of Cambridge
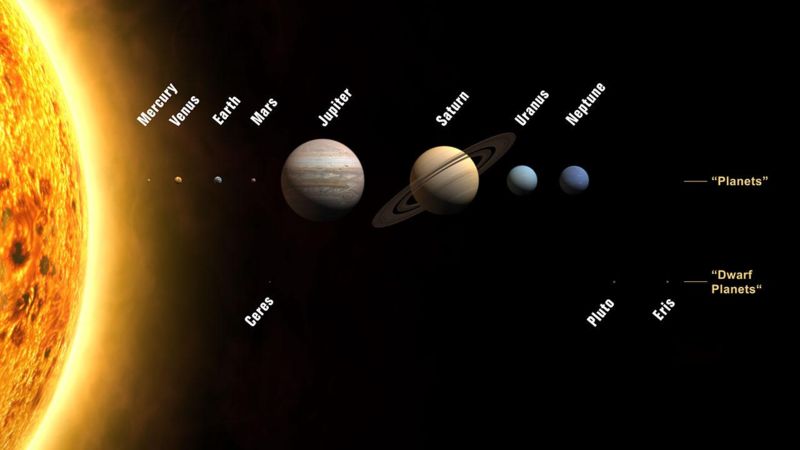 Finally, that moment came. Astronomer Gerry Gilmore joined Ginny Smith to give a very loud demonstration, but first explained what was it that finally made people realise we weren't the centre of the universe...
Finally, that moment came. Astronomer Gerry Gilmore joined Ginny Smith to give a very loud demonstration, but first explained what was it that finally made people realise we weren't the centre of the universe...Gerry - Actually, it's a really fundamental advance in the way that mankind looks at the universe and everything, all the science. We call it the Copernican principle that underlies everything in our modern world. But it really was just a change of attitude. There was no particular experiment, there was no particular new mathematics. The key challenge that people had and it dates right back to the ancient Greeks, as the ancient Greeks said, "Everything in the universe is based on the ratios of integers." And they very quickly discovered that music in particular was based on ratios of nice numbers and shapes that people find pleasing. The architectural columns and so on were based on the ratios of integers. And so, they developed the whole classical architecture and classical music and so on, and realised that this principle underlie everything that our minds found pleasing, and therefore, this must be the way the universe was put together.
Ginny - So, that's ratios of whole numbers that we like.
Gerry - Pi came along and messed it up frankly. But yeah, basically, there are ratios of integers, ratios of whole numbers. So, all the science became a question of looking for patterns. But of course, the fundamental weakness of Greek science, that continued for thousands of years, so about a thousand, 1500 years was that people went looking for the pattern that they thought was nice. And so, that was why fundamentally, science didn't advance. So, people did observe for example the motion of Mars across the sky where you see Mars actually changes direction on the sky and goes back and goes forward again. Now, you can model that mathematically very simply. The Greeks know how to do it. you have a basic circle. But if you need to change direction then you need to seek a little circle that's sitting on top of the main circle which is going the other way. And so, sometimes the two circles are on the right part of the track so that you're going in the same direction and you just go a bit faster than one circle alone and so on. but then some other times, the two circles are fighting against each to other. And so, the planet will go backwards.
Ginny - So, it does a little sort loop the loop as it's going around.
Gerry - It does a little loop the loop. Now, if you try and make a ratio or tone out of a loop the loop, you go, "Mmmm" and it's horrible. And so, they didn't like it. but this process, they're called epicycles. You can build as many epicycles as you like and you get a perfect description from the planetary orbit. So, there's no problem with that. Well, Copernicus came along and said, "Well, I'd like to get rid of these jolts in the music. I want some nice tones" and realised that you get nicer tones if everything was centred on the sun. And so, this is probably the way that nature actually does it. no one took much notice of them but a few people who did ran into serious trouble with religion. It was about a hundred years later that people start taking it seriously.
Ginny - Where did this idea come from that music went with planetary orbits because that seems quite disparate to me, quite different things?
Gerry - That dated right back to the Greeks actually. they were right that the ratios are basic simple numbers do actually describe the orbits as we see them so long as you ignore the second most obvious thing in the sky which is the moon. But nothing much happened after Copernicus until Kepler came along. It was about 100 years later and Kepler said, "Well, I'm going to find those patterns" and realised eventually he found some patterns which worked only if used the sun as the central reference and incidentally, you get rid of the epicycles by using ellipses rather than pure spheres, but that was a sort of detail really. But he found these magic ratios of numbers, so what we now call Kepler's Laws - so the square of the time it takes to go around an orbit is related to the cube as the distance from the sun. And so, that was a ratio of nice integers, 2s and 3s. Everything worked fine and said, "Well, this must be the truth." But his book was called "On the Harmonies of the Seers". So again, he said, "I found a set of patterns which work as musical tones.
Ginny - So again, it's this idea that if something is neat and tidy and sounds nice and looks nice then it's probably right.
Gerry - That's right, yeah. But nonetheless, this idea that, hey, let's just look at what the information on the sky is telling us and take ourselves away from the centre led to the whole of modern science.
Ginny - You've been teasing us a lot by telling us that the planets make these beautiful harmonic sounds. Do you think we can use our audience here to illustrate some of those sounds?
Gerry - Yeah. Well in fact, that's what Kepler actually did. Kepler actually wrote that book called "On the Harmonies of Music" in which most of the planetary orbits were described. And his technical scientific book in which he published the data on the orbits, the planets are represented as musical tones.
Ginny - Are you guys feeling in the mood for a bit of a sing song.
Audience - Yeah.
Gerry - So, let's go. The planets nearest the sun move fastest. And so, they have the highest pitch tone. Of course, what we have to do, we'll speed everything up a bit because it takes the Earth a whole year to go around the sun. If you're just sitting here humming for a whole year, you'll probably get bored and the audience certainly will. So, we're going to speed everything up. So, first thing we need to do then is get the fast moving planet Mercury and Mercury goes whizzing around at a high pitch and so, for Mercury, we're going to have all the primary school children and you're going to sing a tone which goes, "Mmm-mmm" where each one of those ups and downs corresponds a year on the planet Mercury. So primaries, let's do this. And then as soon as you've got going then we're going to go on to Venus, the next planet out and of course, that's for the women in the audience and you do the same thing but with half the range, "Mmm-mmm". Then out to earth the domain of boredom and destitution and going to die and so on. and so, that's role of teenagers. You can come in and do the Earth in a nice hum, "Mmm-mmm". And then we get to Mars which is of course, the male. You can see where these things come from, right? I mean, there's a good logical background behind all these things that we now joke about. So, the men in the audience are going to do the tone for Mars, "Mmm-mmm". Okay, let's practice then.
Audience - "Mmm-mmm"
Gerry - And as Chris introduced, we have these giant minds behind us. So, they're going to be the giant planets and rumble away quietly in the background. So, let's start then with Mercury and the primary school kids. Okay, are you ready?
Audience - "Mmm-mmm"
Gerry - And you keep on going round and round, "Mmm-mmm". Venus cuts in. come in Venus.
Audience - "Mmm-mmm"
Gerry - Okay, Earth, "Mmm-mmm". Good one on Earth. Now, Mars... and the giants. Now what is that if not harmony? Well done.
Ginny - Obviously, it's really useful to know that we go around the sun and not vice versa, but is this actually used in modern astronomy and physics?
Gerry - Well, the most useful everyday application is the fact that we don't actually go around the sun at all. The sun itself moves around the centre of mass, so everything. So, the sun is actually orbiting around a point that's above its surface and we use those wobbles in sun being dragged around by the planets to discover planets around other stars. So, using exactly those harmonies and the very high frequency funny noise that the sun would make and sort of "eeeek" is how we find a planets around other stars.

14:30 - Understanding gravity's pull
Understanding gravity's pull
with Dr Hugh Hunt, University of Cambridge
We are all affected by gravity, every day. It keeps the planets in orbit and  ensures objects fall downwards. But before the 1600s, no one had any idea it existed. Then, in 1687, Isaac Newton published his laws of universal gravitation, and changed everything, as Cambridge engineer Hugh Hunt explains to Chris Smith, with the help of an apple...
ensures objects fall downwards. But before the 1600s, no one had any idea it existed. Then, in 1687, Isaac Newton published his laws of universal gravitation, and changed everything, as Cambridge engineer Hugh Hunt explains to Chris Smith, with the help of an apple...
Hugh - I have 1 Newton. You probably know that an apple weighs about 100 grams and 100 grams is 1 Newton of force.
Chris - Is it true that Newton did fall asleep under this apple tree and had an apple drop on his head and this is how he had the breakthrough intellectually that something is pulling the apple down?
Hugh - No.
Chris - And we can all go home now.
Hugh - In fact, Newton himself said that wasn't true. But the story was told so often that later on in life, he just accepted that it was a good story. So later on, he said it was true.
Chris - What was people's concept of the idea of gravity before Newton came along and actually put some numbers on it then?
Hugh - One of the things that people had trouble with was the idea of a force because everybody thought that things naturally just slow down. If you slide something along the floor, it stops. The laws of motion were that things naturally stop. But then Newton came up with this idea that actually, things will keep moving unless there's a force.
Chris - It is quite groundbreaking sort of idea really isn't it because obviously, here on Earth, everything does stop. So, how did he make that intellectual leap then?
Hugh - Well, he started thinking about motion in terms of rates of change of position and acceleration and he had this idea of momentum. If we think of an apple which drops, it starts off not moving and it gets faster and faster. And it makes that lovely apple sound when you catch it. The idea then is, there must be a force because the apple is accelerating. The thing that he started thinking about was, well, Kepler and others had realised that astronomical bodies orbited. There's a force, the force of gravity. Could the force that's holding the moon into orbit be the same as the force that causes the apple to fall to the ground?
Chris - How is this received by people when he proposed this?
Hugh - Well, he did a nice experiment, a thought experiment which made it rather easy for people to perceive. I wonder if I could get a volunteer from the audience to help with experiment. And your name is?
William - William.
Hugh - Now, what I want you to do is I want you to hold this apple and you just drop it with force straight down. Now, what I want you to do is to throw it towards me. Now, I want you to throw it towards me again. I'm stepping further away.
Chris - We're now about 8 feet away.
Hugh - I want you to throw it harder.
Chris - We're about 10 feet away.
Hugh - Okay now, the further I am away from you, the further you throw it, the curve is less. Now, what Isaac Newton did was to say, "Well, if I throw this really hard, it's going to curve around and it might land somewhere on the other side of Cambridge or throw it harder, it might land in New York. Throw it harder, it could get all the way around the Earth and come back and hit me on the head."
Chris - So, what are you saying is that because the Earth is curving away from the apple because the Earth is a ball, and the apple is falling towards the Earth, if you throw the apple hard enough, eventually, what's going to happen is that the Earth is going to curve out of the way of the path of the apple. So, the apple never actually is going to hit the ground.
Hugh - That's absolutely right.
Chris - So, when we put something into orbit, we are effectively firing or throwing these apples sufficiently hard that it is continuously falling towards the ground but never quite hitting it.
Hugh - And this absolutely transformed the way that we think of motion of astronomical bodies. And the next thing he did was, he worked out an equation which fitted in exactly with Kepler's Laws.
Chris - Let's give William a round of applause. Thank you very much, William. So, tell us about this equation then. What was that equation?
Hugh - For motion in a circle, there is a force required, a centripetal force to cause a motion to go in a circle. It's perhaps best if I demonstrate it really.
Chris - He's getting a brick out of his bag. I'm worried now. It looks like something you ram-raid a shop with.
Hugh - What I have here is - now, let's have another volunteer.
Chris - What's your name?
Lutsi - Lutsi
Hugh - I have a tennis ball and Lutsi, that's a tennis ball. It weighs about 50, 60 grams. It's regular.
Chris - Except it's got a lump of rope going through it.
Hugh - It's got a rope attached to it. Well the rope passes through a tube which is convenient to hold on to.
Chris - It's an aluminium tube with the rope running through the middle of it, ball on one end and a house brick on the other.
Hugh - And I've got a house brick on the other end.
Chris - And the rope is a meter, a meter and a half long?
Hugh - The house brick, it's a heavy house brick, isn't it?
Lutsi - Yeah.
Chris - If you drop the brick, obviously, that's just going to pull directly.
Hugh - There we go. One brick dropped out of the ground and the tennis ball is very light. There's no way the tennis ball can hold up the weight of the brick. Well, I'm going to make the tennis ball lift up the brick by using the acceleration of circular motion, the centripetal force that Isaac Newton talked about when he was thinking about apples and gravity, and the moon.
Chris - How are you going to do that?
Hugh - So, let's see. I'd like you to hold the brick, just flat on your hands. Now, I've got a tennis ball and then I'm going to start swinging it around our heads like this and I want to swing it faster and faster, and faster. And there we go, the house brick is up in the air. The remarkable thing about that Chris, is that we can all see just how big that force is and it makes you realise that when things are going around in orbits around the planets that we might think of gravity as being a quite weak force. But it holds the whole of the universe together.
Chris - So, in other words, that is the demonstration practically of what is actually holding our planets, our little clutch of planets our sun, our moon around our Earth and other planets around other stars in other solar systems.
Hugh - Absolutely right.

21:08 - The explosive history of hydrogen
The explosive history of hydrogen
with Dr Peter Wothers, University of Cambridge
In the 1600s Robert Boyle showed that he could make a gas when he mixed iron filings with an acid, but no-one knew exactly what it was he had made. It took another 100 years for an ex-Cambridge scientist, Henry Cavendish (who actually left the University without a degree!) to figure out that it can combine with oxygen to make water. Chemist Peter Wothers told Ginny Smith about the explosive history of hydrogen...
Henry Cavendish (who actually left the University without a degree!) to figure out that it can combine with oxygen to make water. Chemist Peter Wothers told Ginny Smith about the explosive history of hydrogen...
Peter - Hydrogen had been discovered even before Boyle first prepared it. There was a physician, Van Helmont, who invented the word gas. He noticed these bubbles that come out when you mix acids with different substances and one of them was with metals, just like Boyle later looked at. But Boyle noticed that this gas was flammable. But later, this was studied. This was in 1736 now at the Royal Society, John (Mord) brought some samples to demonstrate in front of his colleagues there this amazing flammable gas. Now, I brought a balloon with me. If I just pick this up, you can probably hear that squeaky. He didn't have balloons in those days. They had to use bladders. Okay, so this was the bladder from an animal.
Ginny - I'm quite glad you didn't bring a bladder full of hydrogen.
Peter - So, he had some bladders filled with this flammable gas and he amazed his colleagues by igniting this gas. And we're going to do this now.
Ginny - Are you sure this is a good idea?
Peter - Apparently, it's never been done here before so I hope the ceiling isn't too dusty.
Ginny - Okay, should we have a countdown everyone? 3, 2, 1... That was brilliant! So, there was a sort of fireball. I could feel the heat coming off it. but it was over very quickly and you could probably hear that kind of popping noise.
Peter - Exactly. So, this was the reaction of course with the hydrogen. Well, we now know with the oxygen from the air. But it really wasn't understood at this time in 1736 what was going on. in fact, there's a lot of confusion between other flammable gases as well. I mean, we're all familiar that we use gas for cooking at home. But that's not hydrogen of course. This is methane predominantly, but this is also flammable. So, these gases were mixed up. I mean, miners knew about flammable gases. They came across methane and so on and terrible explosions. And they compared in 1736 at the Royal Society, samples that had been brought up out of mines with this hydrogen. They noticed the flame is slightly different colour, but they didn't know what was going on. now, we move to Henry Cavendish. He prepared hydrogen in 1766 and he's credited with the discovery because he was really carful about how we prepared and he measured the density of this, and he studied very, very carefully. And he also mixed up different proportions of hydrogen with the air. He noticed that some of them went bang rather loudly. In fact, he worked out the best sort of ratio to get the biggest bang when the hydrogen combines with different volumes of air. But still, he didn't know what was actually happening. A few years later, somebody noticed that they were exploding different amounts of hydrogen in air because it's such fun and then they started exploding hydrogen with oxygen after oxygen had been discovered around 1774. And then they were mixing these proportions together, hydrogen, oxygen getting bigger bangs and then somebody noticed when you do this in a close container that there's a little sort of dew forming around the sides of the vessel. And this was the key observation that then Cavendish picked up on this and he started to study this really carefully and try to measure, are we forming water from these two gases from hydrogen and oxygen. Now, I thought we should try this now and this one that I need a volunteer from the audience.
Freddie - Freddie Osborne.
Ginny - What do we need Freddie to do? We're not going to blow him up like we did the balloon, are we?
Peter - Sort of. But with the balloon, that was just filled with hydrogen. So, there's a nice sort of whoosh, a nice bit of heat. but now, we're going to mix up hydrogen and oxygen and this is rather different.
Ginny - Now, didn't you say that was going to be more explosive?
Peter - Exactly.
Ginny - Freddie is looking a little worried here.
Peter - So, we better give Freddie some safety goggles first of all. You put those one. Okay, so now, Freddie, which hand do you write with?
Freddie - The right.
Peter - The right hand, okay. We'll use your left hand just to be on the safe side there. Now Freddie, I'm going to put into your hand just some - it's washing up liquid. You see if you can keep this in your hand. So, you want cup your hand.
Ginny - So, we're just putting a few drops of nice green washing up liquid into Freddie's hand.
Peter - What I'm doing over here was something that Cavendish certainly didn't do. We are splitting up some water here and this is making just the right proportions of hydrogen and oxygen mixed together.
Ginny - There's a little kind of glass jar with a couple of crocodile clips and then a tube coming out of the top. And then the end of the tube is going into Freddie's hand where he's got a little pool of washing up liquid and that's making lots of nice bubbles of the gas that's coming out of the jar. What's actually going on in this jar?
Peter - So, this is where we're using electricity to split up the water. And as I say, this is something that Cavendish didn't do. He had to prepare his two gases separately and then mix them together. So, he was preparing hydrogen, preparing oxygen, mixing them together. But this is what we've done here. We've just used the electricity to split up the water.
Ginny - So, rather than taking the hydrogen and the oxygen, combining them to make water, we're doing the opposite, just so that we get the right amounts of hydrogen and oxygen for a really good bang.
Peter - Exactly. This is just for convenience here. So, the gas is now collecting in the bubbles here. So, these look like just the normal foam that you would find on the top of your washing up liquid on the bowl when you're doing the washing up. But these are, of course, filled with explosive mixture of hydrogen and oxygen, not normal fairy liquid bubbles at all. Now, we seem to have got quite a few bubbles. I'm just going to put these ear defenders on you Freddie. Can you hear me okay?
Freddie - Yup!
Peter - Okay, right. and now, we're going to explode the mixture.
Ginny - So, Freddie has got a nice handful full of bubbles. Peter is going to light a match.
Peter - Here we go.
Ginny - And just to reassure our listeners, Freddie still has his hand. It's in one piece. And what did it feel like?
Freddie - Not much. I don't really know how to describe it, but it didn't really feel.
Ginny - You can't really anything.
Freddie - No.
Peter - So, all of the energy there was going into the sound really. So, it sounded very loud but yes, it wasn't painful at all.
Ginny - And a round of applause for our brave volunteer. How did this change the way people thought about chemistry? I mean, water is something that's kind of so important and all around us all the time. And this was the first time people really understood what it was made of.
Peter - Not quite actually. they were still reluctant. Even Cavendish was reluctant to really - he didn't fully understand what was happening here. So, what he's learnt is that hydrogen and oxygen can react together and water is formed. But the problem was that of course, there was this ingrained doctrine that the world was made up of just the four elements from the Greeks. This is air, earth, fire and of course, water. So water was thought to be one of the most primary substances and element. And yet, he just showed that these two gases combined to form water. He thought that actually the two gases here, they were modified forms of water. That the hydrogen he thought was - well something he called phlogiston He thought this was phlogiston or maybe water that has too much of this phlogiston . And he thought the oxygen was something that was lacking phlogiston. It was water that didn't have enough phlogiston. And so, when these two things reacted together, water was formed. He really didn't understand quite what was happening. And so, this was the real breakthrough that happened later when the French chemist Lavoisier, finally understood exactly what was happening in this process that these two substances, these elementary substances reacted together to form water. Water then was no longer an element but was actually a compound made up of the elements hydrogen and oxygen.
Ginny - And there's lots of modern science going into hydrogen as a form of power I guess because it can explode like that.
peter - Well, this is so important now because, because there's a lot of energy that's released, as we've seen with the balloons and as we've seen with the bubbles on the hand, a lot of energy can be given out from this reaction as hydrogen and oxygen combine. But the important thing here is that the by-product aside from all these energy is just water. So, unlike all the fossil fuels that we're burning, that would release carbon dioxide that we're now beginning to appreciate isn't necessarily a good thing for our environment, of course, when hydrogen reacts with oxygen, the only product is water. So, this is a very clean energy source.

30:11 - Recreating Volta's Pile battery
Recreating Volta's Pile battery
with Charlotte Connelly, University of Cambridge
We are moving forward in time a few more years now, to a discovery that  changed the lives of countless people. In 1799, scientists discovered a way to generate something that today, we just couldn't live without - electricity. Speaking with Chris Smith, historian Charlotte Connelly assembled a replica of the first ever battery; but she began by explaining what people understood about electricity in the 1700s...
changed the lives of countless people. In 1799, scientists discovered a way to generate something that today, we just couldn't live without - electricity. Speaking with Chris Smith, historian Charlotte Connelly assembled a replica of the first ever battery; but she began by explaining what people understood about electricity in the 1700s...
Charlotte - Everyone knew about thunderstorms and lightning. They knew that that was electricity. They knew about things like electric eels, but most importantly, in 1780s, an Italian called Luigi Galvani had been doing experiments with frog's legs and he discovered that if you take two pieces of different metal and place them at either end of a frog's leg's nerve, it makes the frog's leg twitch.
Chris - Wasn't this the same guy who used to hang frog legs on metal hooks on the metal fence in his garden and he saw them twitching during thunderstorms?
Charlotte - Everyone's got to have a hobby. Absolutely, yeah. He did that after he made this discovery. So, he had seen the twitching. He thought, "Oh, I wonder if this is electrical" and he made the link and then he was doing the experiment with the thunderstorms. And his theory was that there was a special kind of animal electricity that was coming from the frog's legs and was causing this reaction.
Chris - How was that then taken from the concept of animal electricity to the electricity manifest in the environment and then also making electricity on tap?
Charlotte - Well, lots of people got very excited by what Galvani had discovered and one particular person, Alessandro Volta was doing some more experimenting. To begin with, he thought, "Yeah, this animal electricity makes sense." And then as time went on, he began to question it and thought, "Well, maybe it's not as universal as it might be." So, he thought, "Is there a way of getting similar results without using the frog's legs?" he thought there was maybe something about the two different metals. So, he came up with a contact theory of electricity, about contact between two different metals.
Chris - Can you recreate this experiment for us?
Charlotte - I can. So, I've got slightly different ingredients. What Volta did, it was a real kitchen top experiment. He took some silver coins that were in circulation at that time. He got some zinc, cut to the same size because he thought contact between two different metals, that's important. He placed one on top of the other and then he thought, well, there was some fluid in the frog's legs. So, he took some cardboard and made it damp. He found it worked better with salt water.
Chris - So actually, just looking at this, what we have in front of us are a whole load of squares. These are an inch, an inch and a half square pieces of - what metal is that one? That's quite heavy. So, that's zinc. That's a silvery colour and then we've got these coppery coloured sheets. Are they copper?
Charlotte - They are copper. Copper is rather cheaper than silver and lots of people later on went to use copper.
Chris - And then we've got these things that look like the kind of thing you'd put on a wound in the hospital. They are the things you put on a wound.
Charlotte - Precisely. They happened to be exactly the same size and unlike cardboard, they don't stick to the zinc.
Chris - So, these are Volta's bits of cardboard.
Charlotte - Exactly. So, we take a piece of zinc, we place that down. And then because we've got a slightly more advanced theory than Volta, we put our bits of cardboard in between the two. So, we first dip it in our saltwater and we place that on top.
Chris - And so then what we're going to do is put on top of the cardboard piece a piece of copper.
Charlotte - That's right. So, we have a layer of three things, zinc, cardboard, copper and then we carry on, we do it again - zinc, cardboard, copper, zinc, cardboard, copper.
Chris - And here's one magically you made earlier. This is quite big. This is 6 inches high of these.
Charlotte - This is a 20-cell layer. So, I've done that 3 layers 20 times over.
Chris - Scientifically speaking, what's actually going on in each of the little layers in the battery?
Charlotte - So, in each cell, you get some free electrons that move by potential energy to one side of the surface so from the zinc to the copper. And because we've got lots of them piled up, that happens more so you get - in today's terminology - higher voltage and no marks for guessing where voltage comes from, given that we're talking about Alessandro Volta. So, the greater your pile, the more layers you have, the more voltage or electromotive force you have. And then what happens when you create a circuit is all of that potential energy discharges and you get a bit of a sensation which I think we should probably get someone to experience.
Chris - Who would like to volunteer to help us with our battery? What's your name?
Amelia - Amelia.
Charlotte - We're going to get Amelia to put one finger from each hand in the saltwater. So, she's a really good conductor. Now, with your left hand, I would like you take this piece of zinc wire which is attached to the bottom piece of zinc in the pile. With your right hand, I'd like you to touch the very topmost piece of copper.
Amelia - It felt like a static electricity shock which I just had twice.
Charlotte - Very good and I think we can get a stronger shock if you're feeling brave, do you think?
Amelia - Yeah.
Charlotte - So, I'm giving you a piece of copper to take in your right hand. So, Volta discovered that if you touched the pile with pieces of metal that he got a stronger shock. So, same again.
Amelia - It did feel much stronger.
Charlotte - Very good. So Volta didn't have a way of measuring how much electricity was coming out of these things. So, he said things like, "I got a shock up to my knuckle or a shock up to my wrist." Do you think that would be a good way to describe it? Did it feel like it went further?
Amelia - Yes, it did.
Chris - Thank you very much, Amelia. Is there any way we can measure this or prove that we are actually making some electricity so people at home believe us?
Charlotte - Well, I have got a buzzer and if I attach it, hopefully, it's going to make funny noise. Now, I'm just going to dip the ends in our saltwater to help it conduct a bit. So, if I take the negative side and attach it to the zinc and the positive side and attach it to the top, (beep).
Chris - No. the fire alarm has not gone off. It is working. Charlotte, how did this change people's perception of electricity and also, what did it enable them to do once they could make cells like this and batteries like these voltaic piles we've created here?
Charlotte - Well, it took off really quickly because it was something you could do at home with really simple equipment. So, loads of people started experimenting and within weeks, a couple of people, Nicholson and Carlisle were their names, took a pile like this, stuck their ends in water and found out that it gave off hydrogen and oxygen which as we learned earlier, if you put them together, you get water. And this led to all sorts of experiments and a new discipline called electrochemistry. So, Humphrey Davy was famous for having a massive pile. In 1807, so only 7 years later, he started coming up with all sorts of new elements that he isolated and potassium and sodium were the first ones.
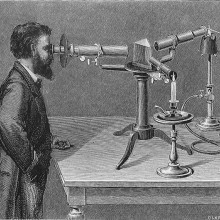
36:45 - Seeing the stars with spectroscopy
Seeing the stars with spectroscopy
with Dr Josh Nall, Whipple Museum
No one has ever been to the Sun, so how do we know that it's over 90% hydrogen gas with some helium, oxygen, nitrogen and a few other elements? The answer lies in the light that the Sun sends out. Speaking to Ginny Smith, Cambridge historian Josh Nall demonstrated a device that early scientists used to study this light: the spectroscope...
Josh - It's actually a spectroscope from the mid-20th century which hopefully, we can get someone up here to have a go with.
Ginny - What exactly is a spectroscope?
Josh - It's an instrument for looking at the spectrum of light. As we've known for a long time at least, since the time that Isaac Newton was working in this town, when you shine light through a prism, the prism refracts that light which is to say it splits it up into its constituent spectrum.
Ginny - So, this is why you get rainbows because the water droplets split up white light into all the different colours and we call that a spectrum.
Josh - Precisely.
Ginny - How can this help us find out what something like the sun is made of?
Josh - I think the easiest way to explain it here to our audience is, to begin with actually, our most modern instrument that we have here, a modern computer spectroscope.
Ginny - So, we've got a screen here with a kind of graph at the bottom and a rainbow at the top. It's connected to a long tube which has a thing in the end which I guess is the spectroscope. So, if you point this at different things, will we see different kinds of rainbow on the screen?
Josh - Indeed. So, what we've got here on the graph at the bottom is the wavelength of the light that is coming into the end of this tube and then we have intensity here. We can actually visualise at the top here the colours. So, if we shine white light we start to see pretty much contributions coming into the spectroscope from every part of the spectrum. This is what Isaac Newton first proposed was that actually, white light was composed of these more fundamental colours. We're now looking in the mid-19th century into scientists who start to want to investigate whether by looking at the nature of light, one can actually determine what substance it is that's giving off that light.
Ginny - But they didn't have a nice computer screen and a nifty little gadget like this. So, what did they have to do?
Josh - Crucial to the studies were two German scientists - Robert Bunsen of the Bunsen burner fame and Gustav Kirchhoff. One of their crucial insights was that they began to look at the spectrum that individual elements gave off. For some time, people had been aware that when you looked at the light through a prism that came off of substances, you started to get certain colours very prominently appearing in bright bands and certain colours not appearing at all.
Ginny - So, the same reason why if you've done that school chemistry experiment where you put different elements in a flame, they burn different colours, they also give off different coloured lights and you can pick that up with a spectroscope.
Josh - Absolutely. And so, they started to think, well maybe then we can actually use the colour of light to determine what different elements are giving off that light. So, if we take a specimen, a modern specimen that we have here as an example, this is a tube of neon. As many people will be aware when you pass a high electric voltage across a tube of neon, it will glow a kind of characteristic colour.
Ginny - Does anyone want to come and give us a hand? What's your name?
Minnie - Minnie.
Ginny - You can see the neon here and it's shining a beautiful pink colour. So, what we need you to do is just point the spectroscope at it and tell us what you can see on the screen.
Minnie - Well, it's red colours and yellow and orange.
Ginny - Exactly. Is that what you'd expect?
Josh - Yeah. So, you're starting to see very specific peaks at certain specific points in the spectrum. So, what I want to do here with our volunteer is see if we can recreate this with natural historical objects. So for this, we're going to have to get you to put on some gloves.
Ginny - So, this is kind of about the size of a lipstick. You could quite easily slip it in your pocket and you can look through one end and at the other end, is there still a prism in it?
Josh - There's actually a series, a chain of prisms throughout the tube which are positioned against each other so that the light passing through will be split into its spectrum by the time that it reaches your eye. You want to hold it and look down there.
Minnie - It's a rainbow.
Josh - Can you see any colours in particular, any prominent colours there?
Minnie - There's still this red, orange, yellow, lots of green, blue and purple.
Josh - What happens as you said there is you get many multiple bands. But Gustav and Kirchhoff noticed was that certain bands were particularly prominent. These, they could use as a kind of fingerprint for a given element. The next crucial discovery was to think about sunlight because people had noticed for some time when they were working that the spectrum that you get from sunlight has this continuous rainbow effect. But then it has this thick black bands across the spectrum and nobody really knew what these black bands were. And Gustav and Kirchhoff realised that you could map up the fingerprints of individual elements to these black bands in the spectrum of the sun.
Ginny - So, rather than seeing those bands standing out, they'd actually being kind of taken away.
Josh - Yes. So, this is what's called an absorption spectrum. What happens when sunlight passes out from the centre of the glowing sun, it actually has an outer atmosphere around it. as the light passes through that atmosphere, the elements in that atmosphere absorb at bands that are specific to that particular element.
Ginny - So, you can tell what that light has passed through on its way from its source to you.
Josh - Absolutely. The crucial discovery here is for the first time, you could actually chemically analyse the sun and you could determine what elements there are present in the sun.
Ginny - How did this change the way people thought about science or about the universe?
Josh - Well, I think it had a really profound impact on people's outlook on the nature of the universe because this discovery was made around 1859. And not that long before, in the 1840s, you've got people like August Comte writing a book in which he claims that it will be impossible for man to ever know what the sun is made. It would be pointless even to try studying because it's so far away. Ten years later, you've got William Whewell here in Cambridge, gets into a heated debate with other philosophers about whether or not there's life out there in the universe. And Whewell points out that there can't be possibly be life out in the rest of the universe because we don't even know whether the universe is made from the same stuff as we find on Earth. But then in the wake of these discoveries of the spectroscope, you've got proof that matter out in the universe is composed of all of the same fundamental elements as the materials that we find on Earth. Not just that the sun contains elements that we're familiar with, but crucially, that you can start to find substances like iron and water on planets like Mars which were discoveries that were soon made. That starts to make people go the other way to say, "Well, hold on. the universe might be full of planets just like the Earth." The other use of the spectroscope which we haven't mentioned is that chemists were also using them to actually discover new elements. So, a lot of the work that the spectroscope did in the hundred or so years that followed Bunsen and Kirchhoff's work was actually to discover new elements. Because of course, if you find a signature which you can't map to an element you already have, it's quite possible that you have new elements. So for example in 1868, Norman Lockyer discovers helium in the corona of the Sun.
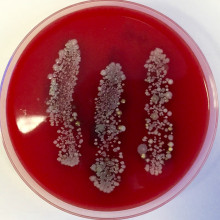
44:54 - How many bacteria live on your hands?
How many bacteria live on your hands?
with Dr Michael Nicoll, University of Cambridge and Rachel Thaxter, Addebrooke's Hospital
A recent survey of commuters' hands showed that up to 60% of travelers have faecal bacteria on their fingers. Women, administrators and people who travel by bus emerged as the worst culprits. You might hope that your doctor would have cleaner hands - and these days handwashing is a major priority - but it 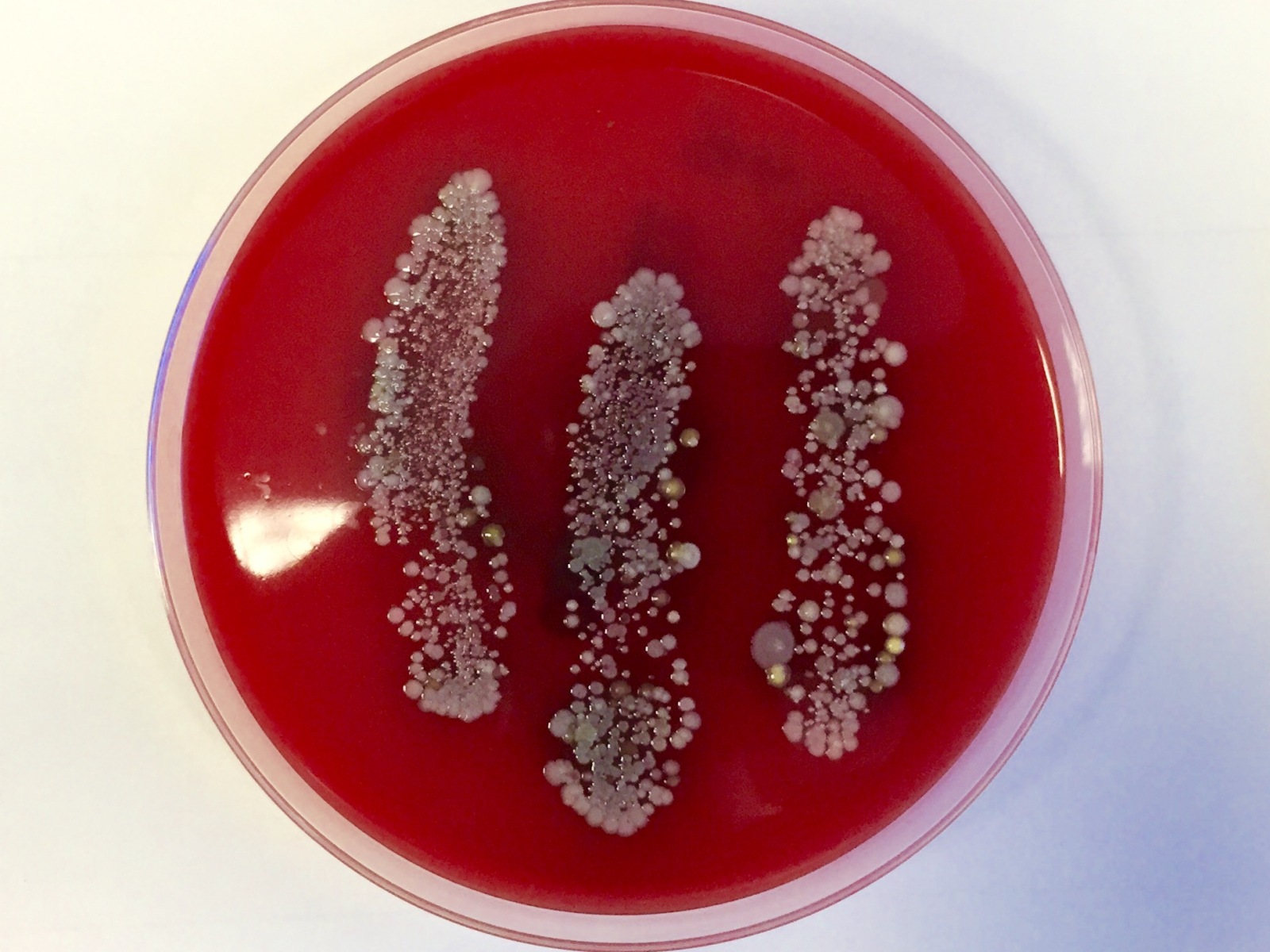 wasn't always like that. In fact, it took the Hungarian doctor Ignaz Semmelweiss in the 1840s to make the link between doctors doing post mortems in the morning - without washing their hands afterwards - and a big surge in patient mortality in the afternoon!
wasn't always like that. In fact, it took the Hungarian doctor Ignaz Semmelweiss in the 1840s to make the link between doctors doing post mortems in the morning - without washing their hands afterwards - and a big surge in patient mortality in the afternoon!
But how effective is hand-washing, and what's living on our mits anyway? Chris Smith spoke with Michael Nicholl, a virologist at Cambridge University, and Rachel Thaxter, an infection control,nurse at Addenbrooke's Hospital...
Chris - So, you have brought along some Petri dishes, a fetching green colour and they have some red stuff and some dots. Tell us more.
Mike - So, because we don't want to recreate the experiment that Ignaz Semmelweis carried out, today, we're going to do a couple of demonstrations of the bacteria that live on the hands of the Naked Scientists' interns.
Chris - Tell us what you did to create those plates.
Mike - So, these are blood agar plates and they form like a food layer for the bacteria to grow on. What we did is we went and got the hands of the Naked Scientists, put them on the plates before hand washing and then one of them washed their hands and put it on the plate again, and the second person washed their hands but with an alcohol rub, like the stuff that you find in hospitals.
Chris - So, we're looking to see if standard hand washing versus alcohol based hand rubs would make a difference to the levels of bacteria because those little dots we're seeing, all kinds of nice colours and things growing on the plates, those are colonies of bacteria.
Mike - We have bacteria like everywhere on our bodies anyway, but throughout the day, we also pickup various other nasties. So, what we have here are the hands before they were washed, very nice plate here. You can see exactly where the three fingers of the intern were scraped across the plate.
Chris - So, what are those bugs?
Mike - These are very likely to be species of Streptococcus and Staphylococcus which live on our skin normally. Staphylococcus aureus is related to MRSA. Streptococcus is used in yogurt quite a lot and certain strains such as Streptococcus pyogenes are likely the cause of the disease on the ward that Ignaz Semmelweis actually treated.
Chris - So, the doctors doing the post mortem would've chopped up somebody who had died with this infection and got the bugs in increased numbers on their skin. When they went to see the patients in the afternoon, then they would've infected them with those microorganisms causing more customers for the post mortem room the next day.
Mike - Absolutely. It was interesting because the reason the thought occurred to him that there was an autopsy link was a friend of his was accidentally cut himself with a scalpel during an autopsy. He developed septicaemia, an infection of the blood. When he did the autopsy on his friend who dies as a result, he noticed that the lesions on his friend were the same as these women in the maternity ward. And so, he thought something is happening. Something is being transferred from the bodies, the decomposing bodies of the dead and being given to these women which is causing the disease. It was a huge breakthrough because at that time, people thought, to get sick, you got struck down by invisible poisonous gas called miasma. Which if you think invisible poisonous gas will hit you, there's no way of preventing that, you have no idea. So, people felt very powerless whereas when you actually control infection by hand washing which you did with the chlorine solution on the doctor's hands, you can actually say, "We can prevent disease being spread." There must be something more to it or something different of course than an invisible poisonous gas which will kill us.
Chris - And talking of hand washing, did it make a difference to our interns?
Mike - It did and they should wash their hands more often. When you look at the hand washing, you can see there's far fewer. There are still colonies on there but the job is pretty good. When you compare this plate here and compare it with the alcohol rub, you can see that this has killed most of the stuff that would live on hands.
Chris - Indeed. This plate which is from the hand after it's been cleansed with alcohol, that it's almost devoid of any kind of growth. It's almost sterile.
Mike - Absolutely. So, the alcohol will kill the bacteria that's sort of very much attached and colonising your hands anyway as part of your normal skin flora.
Chris - It seems extraordinary Mike, they must have seen these sorts of things growing. They must have seen for instance, bread going mouldy. So, how do people account for that then if they didn't have a theory or a concept of microorganisms?
Mike - People didn't know at all where it came from. In fact, most people thought that mould on bread was spontaneously generated. It would just come out of the ether. The experiments that we're talking about it occurred in the 1840s, but it would take about 20 to 30 years later for Lister to show that actually, when food is spoiled, or when wine is spoiled as he was doing, it was actually...
Chris - More important the wine, isn't it?
Mike - I mean, wine exactly. When the wine is spoiled, it's because the bacteria were falling into it and actually having a great time in the wine.
Chris - Thank you very much Mike Nicoll from Cambridge University. Because at the main stay of defending our health in hospital is good and immaculate hand washing, we thought we would invite one of the infection control team from Addenbrookes Hospital Rachel Thaxter to come and give us a lesson and also, show us how good or bad we are it. Please welcome Rachel Thaxter. So Rachel, you're on the war path after any doctors and nurses who don't wash their hands properly. How much of a difference does it really make? I mean, we've seen some plates here with some cultures on, but are they the kinds of bugs that really make a difference?
Rachel - They are certainly, yeah. As Mike said, Staphylococcus, Streptococcus, we have these things on us and they do cause real problems in some patients. But it really is what you do with your hands that is the problem and your technique of washing your hands.
Chris - You've brought with you this thing. I love this. Glow and Tell. Tell us all about it.
Rachel - I'm going to ask a volunteer to come from the audience.
Chris - Let's welcome them up. What's your name?
Lucien - Lucien.
Rachel - I'm going to squirt this cream into your hand, I want you to rub it in like a hand cream and then have a look at your hands under the UV box and see how much you've got on your hands to make sure you've got good coverage. I want you to go to the bathroom and wash your hands like you would normally at home, then come back and we'll have a look at how you've done with the UV box.
Chris - Right. So we're putting them under the UV light and actually, you can see on Lucien's hands, it's glowing bright blue.
Rachel - Chris, I've got some on my hands as well. I shook your hand. Let's see how much you've actually got on your hand.
Chris - Right. so, I've literally just shaken hands with you. Look, all my fingers are glowing up actually.
Rachel - So, our volunteer has come back now. Let's see how they've got on. Not too bad actually Lucien. You just see. You're actually quite good at washing this hand here. You just missed a bit with your wrist there and your fingertips, I can see the very edge of your fingertips where your nails are. Are you right-handed Lucien?
Lucien - Yeah.
Rachel - So, you can see that actually you washed your left hand much better because your right hand is the dominant hand.
Chris - The bottom line is, there's still lots and lots of that dye. So, were those microorganisms that would be potential sites for transmission of infection. Rachel, thank you very much. Rachel Thaxter from Addenbrookes Hospital.

51:56 - How do our memories work?
How do our memories work?
with Dr Michelle Ellefson, University of Cambridge
Psychology is a relatively new science; it wasn't separated from philosophy until the 1870s, when people realised that it was possible to conduct experiments in psychology, just like in the other sciences. One of these early experiments was conducted by Herman Ebbinghaus, who wanted to test how  good his own memory was. With the help of Ginny Smith, Michelle Ellefson tried out the kind of experiments he used on our audience...
good his own memory was. With the help of Ginny Smith, Michelle Ellefson tried out the kind of experiments he used on our audience...
Michelle - So, you're going to do a list of items. I'm going to name them off one by one. Just like Ebbinghaus's experiment where he actually learned these 3-letter words. They were nonsense words. He learned them himself and he measured how well he could do at remembering them later. So, we're going to get everyone to participate. So first, you just have to try to remember as many of these as you can. Alright, 1- MQK, 2-PCH, 3-RLV, 4-JPF, 5-XDT, 6-VKM, 7-FTG, 8-QHN, 9-SNB, 10-LJC.
Ginny - Do you think you got them all? No? I'm seeing some quite pained looks around the room. People find that quite difficult? Yeah?
Michelle - Would you believe that he learned over 84,000 of these throughout his experiment? He studied them for over 800 hours.
Ginny - That's dedication.
Michelle - So, we're going to see how many the audience might be able to remember. Do you remember what the first one was?
Ginny - Hands up if you think you can remember it. There's probably 10 here in a room of maybe 50.
Michelle - Shout it out if you think you know it.
Ginny - Okay, on the count of three - 1, 2, 3...
Audience - MQK.
Michelle - Good. How about the last one?
Ginny - Hands up if you remember it. Okay, we've got 3.
Michelle - It's a smaller number than the first one. So, what do you think the last one was?
Audience - JLC?
Michelle - Close. There's few mixed up letters, but LJC, that's pretty good. Okay, so what about number 6?
Ginny - We've got no hands up. So, why is it so much harder to remember the ones in the middle than the first one or even the last one?
Michelle - One of the reasons why the largest number of people in the room remembered the first one is because as I was going through the list, it was the first one that you were kind of rehearsing in your mind. So, you rehearsed it more times than any of the others. As you got to about the third or the fourth item, because these items don't really have meaning for us, then it just started to be a jumble. The last one, people remember was a little bit higher rates because it's the last one you've heard. So, what this experiment demonstrated was something that people called the serial position effect
Ginny - Why was this such a historically important experiment?
Michelle - The first one is that no one believed that we could measure higher order thinking, just our general thinking skills in an experiment. Everyone before this point had thought about thinking skills and cognition through thought experiments and philosophy. But nobody thought we could actually do an experiment to measure our thinking skills. The second reason why it was really important was that while Ebbinghaus was conducting this research, statistics were just coming on the scene. He in his seminal work took lots and lots of statistics. So, he unfortunately did all this work on himself which is a criticism, but he took averages. So, he have these lists of 10 items and he'd have an average of how long it took him to learn a list. And how easy it was to forget a list. If you want to go back and relearn it, how quickly he could relearn it. So, he had lots and lots of statistics and he used a lot of terms that psychologists hadn't really been using before to that extent.
Ginny - Is this something that we can use to help ourselves learn better to help children learn better?
Michelle - So, one of the things that Ebbinghaus did in his learning of these various lists, he engaged in what people now call distributed practice, which is doing a little bit often. What a lot of people who study cognition and learning now think is that that's one of the best ways to learn. So, we might think that it's a lot easier to learn a bunch of stuff if we block learn it, which means we focus on one thing until we know it. But actually, in terms of memory, we do a lot better if we can space out our practice and do a little bit every day.
Ginny - So, there's a real reason you shouldn't just cram for exams.
Michelle - Yeah, you won't remember it.
Ginny - Now, did we want to see if anyone here has got a really good memory and can still remember any of those word lists?
Michelle - So, shall we start with one? Since you now know one is maybe the easiest, does anyone remember what the first item was?
Ginny - We've got a couple of, a few hands. I think it's less than the first time.
Michelle - It's less than before. So, that's another one of Ebbinghaus's results from his finding is this idea that the longer you go from learning something, the more you forget. This later became called the forgetting curve. So, in relation to examinations, the reason why this thing called distributed practice works is because just as you're getting ready to forget something, you remind yourself what it is. So you might do really well in an examination if you cram for it the night before. But two days later, you won't remember much.
Ginny - So, you need to do little and often and then cram as well- that's the worst of all worlds! So, who thinks they can still remember number one?
Audience - MQK.
Michelle - Very good, but I'm sure no one remembers number 7. You think you remember 7?
Audience - TMG?
Michelle - Close but not quite. It's FTG.
Ginny - So, if people do remember a random one, is that likely to be because it means something to them? If it was say, your initials, you'd remember it.
Michelle - Yeah, so I tried to be really careful in creating these sets of letters to try not to have something that was meaningful. But that's another part of memory is that the more meaningful something is, the easier it is to remember.
Ginny - Fantastic advice. I'll remember that next time I'm doing an exam although hopefully, that won't be any time in the near future. That was Michelle Ellefson from Cambridge University.









Comments
Add a comment