The World needs energy, and if we want to keep the climate stable, then it had better be clean energy. At the same time, the Sun is bathing us in thousands of times more energy than we currently consume, and we know how to turn the Sun's energy into electricity with solar cells.
energy than we currently consume, and we know how to turn the Sun's energy into electricity with solar cells.
But, the solar cells on the market today, although they are getting cheaper, are far from being as efficient as they could be in terms of their ability to turn light into electricity. In fact, the efficiency of the best cells is significantly less than 30%.
To address this problem of poor efficiency, we first need a crash course in what light is and how a solar cell works. Light consists of tiny energy particles called photons. For example, red light consists of "red" photons with a specific amount of energy. When these photons hit the solar cell, electrons inside the cell get knocked loose and, tapped off into a circuit, it's these electrons that give us the current.
But in order for a photon to knock loose an electron, it needs to have a minimum amount of energy. On the other hand, if the photon packs a bigger energy punch than this minimum, the surplus is just wasted as heat. And here lies the problem.
Sunlight contains photons with a wide range of energies. It can be divided into three parts. The visible light we can see; the high energy ultraviolet (UV) light that, amonst other things causes sunburn, and low-energy infrared (IR) light, which we experience as heat.
For silicon solar cells, red photons have just a bit more energy than is needed to knock loose an electron. Red photons are therefore used very efficiently by such silicon-based cells. On the other hand, blue and UV photons have much more energy and could knock loose two or even three electrons per photon respectively. But since we lose all the surplus energy from these high energy photons as heat, the efficiency is cut in half for blue light and cut to a third for UV light. Lastly, we have the low energy part of the sunlight. The IR photons, or heat rays, have less energy than red photons. Most of them don't have enough energy to be used by the solar cell and pass through it like visible light passes through your house window.
So the efficiency of a silicon solar cell to convert red light into electricity is extremely good, even for poor cells. But the efficiency then drops steadily with increasing photon energy. At opposite end of the scale, the efficiency is zero for most of the IR part.
This is why the solar cells today are only around 20% efficient. Had the sun shone pure red instead of the full spectrum, the energy crisis would 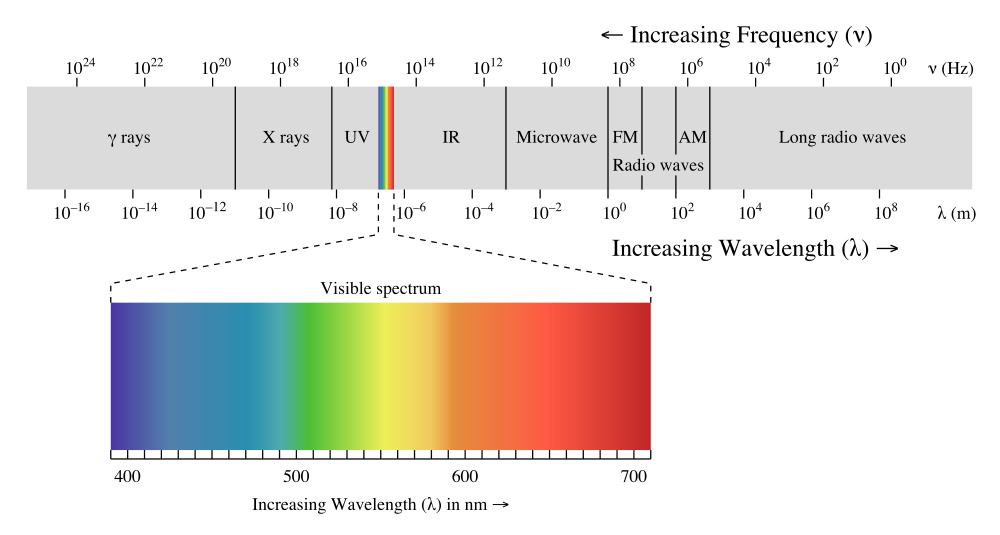 have been solved years ago! But what if we could just turn the sunlight red?
have been solved years ago! But what if we could just turn the sunlight red?
This is not as far-fetched as it might seem. In fact, all you need is something that absorbs the light that you want to change, and something that shines with the colour you want. And we have been doing this for decades. Fluorescent lights contain a UV light source (mercury atoms in a vapour inside the tube) and the white coating inside the glass converts the UV light into visible light. Optical brighteners used in laundry detergents and paper also utilise this effect, converting UV light into blue light to make your clothes appear shining white.
This is a one-to-one conversion (called down-shifting), turning one UV photon into one visible photon. But solar cells will not benefit much from doing this, because the extra energy is still being lost as heat. Instead what we need is a one-to-two conversion achieved by splitting one high energy photon into two medium energy photons (called down-conversion), and a two-to-one conversion by combining two low energy photons into one medium 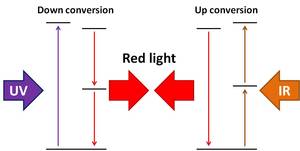 energy photon (called up-conversion). Up- and down- conversion are a lot trickier than down-shifting. The difference is that while laundry detergent only needs to absorb light and reemit it at a different wavelength, new solar materials will have to include at least one extra step to either split a photon in two or combine two together. And this complicates everything. A lot.
energy photon (called up-conversion). Up- and down- conversion are a lot trickier than down-shifting. The difference is that while laundry detergent only needs to absorb light and reemit it at a different wavelength, new solar materials will have to include at least one extra step to either split a photon in two or combine two together. And this complicates everything. A lot.
Materials already exist that can convert one high energy photon into two lower energy photons. But, unfortunately, they either require photons of an energy that don't exist in natural sunlight (like X-rays), or they aren't very effective. Materials that combine two low-energy photons into one higher-energy photon also already exist. They're used, among other things, for marking proteins in biological specimens. But these materials require high-intensity monochromatic light, like a laser, so they won't work with broad spectrum sunlight either.
Luckily, we know of many materials that absorb and emit light with colours ideally suited for solar cells. For example, we could take the UV-absorbing part of sunscreen (titanium dioxide) and mix it with the chemical used to make red spots of light on TV screens (europium) and we get a material that down-shifts one UV photon to one red photon.
Now what we need is the middle part, the photon combiner or splitter, to make an up and down converter. There aren't as many candidates for this, but there are a few. The lanthanides (like europium), which are also called rare-Earths or f-metals, is the class of materials that does this best today. The problem with the lanthanides is that they only deal with a very narrow range of photons at a time; for example one compound can convert 980 nm photons, but not 950 nm photons. The transition metals, or d-metals, like iron or titanium, can deal with a larger range of photons, but they are not very efficient at splitting or combining them. There are other types of materials as well, like organic or carbon compounds, which absorb photons well and can be quite cheap, but they can rarely split or combine photons and usually degrade over time.
The challenge is therefore twofold. First we have to find a good middle component, the splitter or combiner, which is compatible with the other two parts of the system, the absorber and emitter. Secondly, we have to put together the parts in a very exact and efficient manner. This will almost certainly require us to control the chemistry at the atomic level, because many complex materials like this rearrange themselves spontaneously into configurations other than the ones we want to if we let them.
But we;'ve come a long way. We now know how to make efficient down-shifting (one-to-one) materials and we are starting to have quite a good idea of what we need for up- and down-converting materials as well. And, through nanotechnology, we have also discovered new ways to control materials at the atomic level. So with a lot of hard work and maybe a little luck, we will manage to put together efficient light conversion materials for solar cells.
When we manage to make these conversion materials, they don't need to be physically connected to the solar cell itself. It could be put on top of the solar cell, but it could very well also be put on top of the glass or 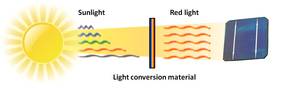 plastic cover in front of the cell. In addition, all silicon solar cells are very efficient at converting red light into electricity, both old and new. This means that not only can we potentially double the efficiency, we can also use it on the existing solar cells we already have today. We can even imagine buying these new materials in spray cans to simply spray onto our old cells. Efficiency in a can, so to speak. When we manage this, the potential is to double the efficiency of solar cells and make solar energy not just clean, but cheap and efficient too.
plastic cover in front of the cell. In addition, all silicon solar cells are very efficient at converting red light into electricity, both old and new. This means that not only can we potentially double the efficiency, we can also use it on the existing solar cells we already have today. We can even imagine buying these new materials in spray cans to simply spray onto our old cells. Efficiency in a can, so to speak. When we manage this, the potential is to double the efficiency of solar cells and make solar energy not just clean, but cheap and efficient too.
- Previous Bigfoot: The Nitrogen Problem
- Next Mushroom Magic










Comments
Add a comment