Ingredients
It is possible (Though very dangerous so please don't try it) to build a relatively simple laser which works on the nitrogen in the air atmospheric air.
The idea is to very rapidly apply a very large voltage between two plates, this excites the air between them and lasing occurs between them.
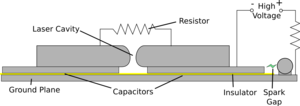 |
| The basic setup |
I had more trouble than I was expecting getting this to work, and I had to reduce the pressure a bit using a vacuum pump which increases the lifetime of the excitied molecules, and makes the whole thing a little easier. It didn't seem to lase every shot, but did sometimes.
| |
| TEA laser firing |
The Basic idea
The air molecules are excited by the high voltage, some of them give off this energy as light, which triggers others to, creating a cascade of light being emitted.
 | The air molecules start off unexcited. |
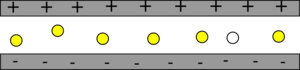 | A very high voltage is applied very quickly, exciting most of the air molecules. |
 | Some of the air molecules spontaneously emit a photon of light. This then hits another molecule stimulating the emission of a second photon, which hits more molecules and stimulates more light. |
What is the circuit doing?
The circuit is basically there to apply the high voltage very rapidly
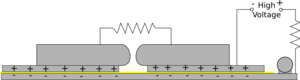 | 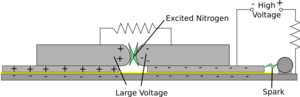 |
| First of all the high voltage charges up both top plates to about 10kV | The spark gap then breaks down effectively shorting the right hand plate to the ground plane. This produces a very rapid voltage difference between the two plates. |
Why do the atoms Lase?
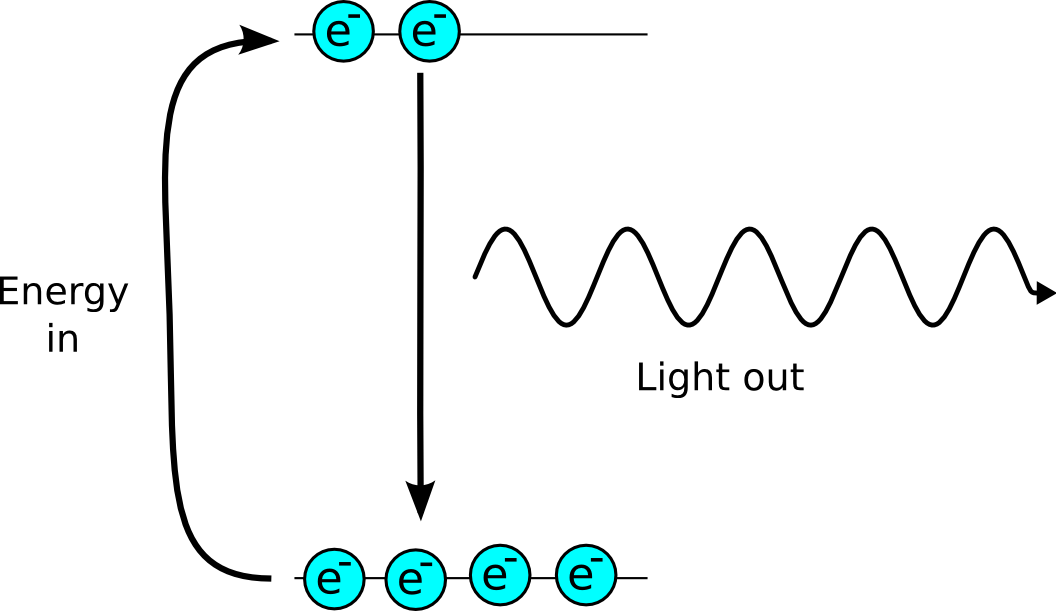
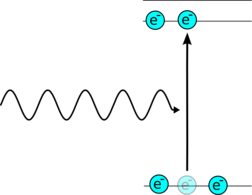
In an atom electrons are constrained to certain energy levels, an electron can absorb energy an move up the energy levels. It can then emit this energy in the form of a photon of light. It can do this spontaneously when the light is emitted randomly, or it can be stimulated to release the energy by another photon of light. The stimulated photon is exactly the same in direction frequency and phase as the original photon.
 |  |
|---|---|
| An excited electron can spontaneously emit a photon of light in a random direction. | An excited electron is hit by one photon of light which stimulates it to emit a second identical photon. |
Laser stands for Light Amplified by Stimulated Emission, creating some photons of laser light is quite easy, but to amplify the light is more difficult.
The problem is that electrons at a lower energy level can absorb light, so if there are more electrons at the bottom of the transition than the top, the electrons will absorb more light than they will emit.
Unfortunately for the laser designer naturally there are more electrons at the lower energy level, as some get there by loosing energy to heat, or by spontaneous emmission.
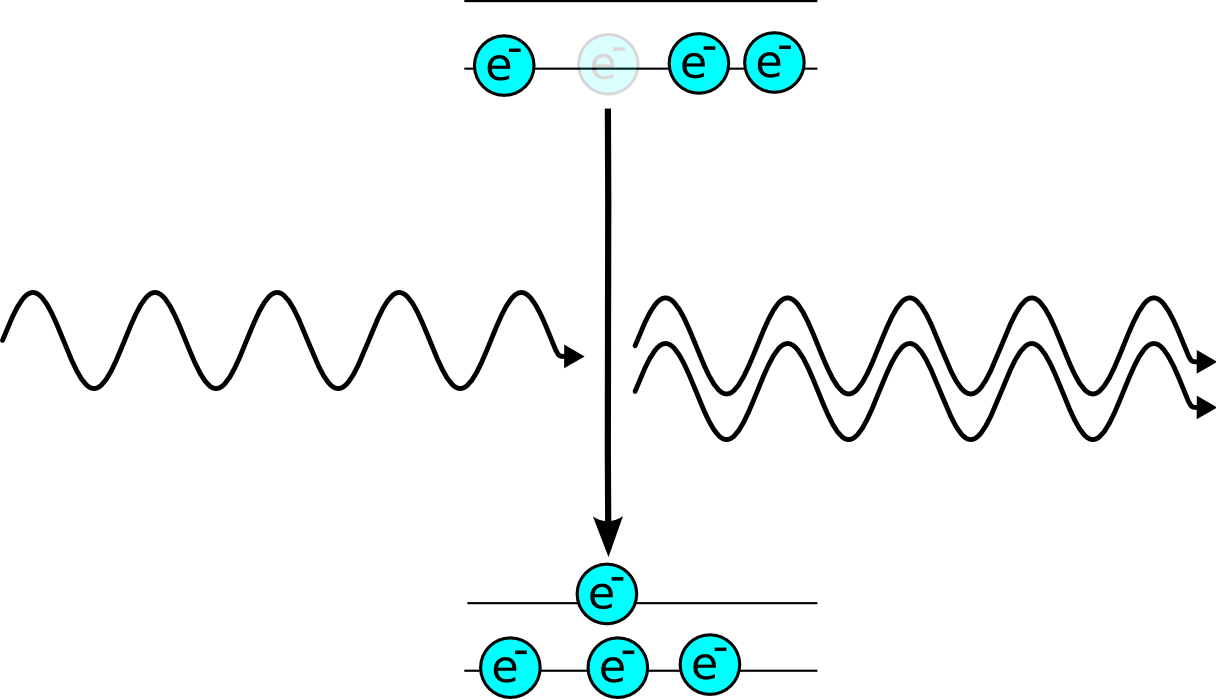
The way around the problem is to use a more complex energy level structure. If the emmission of light puts the electron into a state which it leaves very quickly to reach the ground state. This means that there are more electrons at the higher energy level than the lower one, which is known as a population inversion.
 |  |
| An electron in the lower state can absorb energy if it is hit by a photon. | To avoid this problem lasers are built with a very fast transition from the lower level to a ground state, which keeps the lower level empty |
This means that a photon moving through the lasing medium is more likely to be amplified than absorbed so you get a laser.
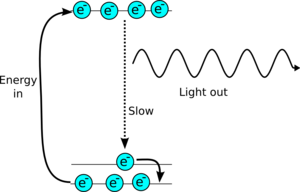
The nitrogen laser is active for such a short period of time because the top energy level has to be easy to get into, so it is also easy to escape by spontaneous emission. So the lasing state only lasts for a few tens of nanoseconds. This makes building the laser very difficult, as you have to excite all the air in the cavity within a few ns of one another.
More sophisticated lasers use systems with 4 states and a lasing transition which is very bad a spontaneous emission, and so the laser can keep on emitting continuously.
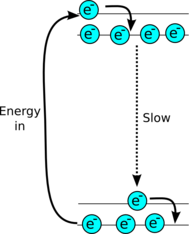 |
|---|
| More sophisticated lasers use a 4 level system so the lasing transition can be very slow at spontaneously emitting energy, so they can keep on lasing. |










Comments
The detailed instructions for
The detailed instructions for this laser can easily be found in the Internet. What makes you say this laser build is dangerous? Is it the high voltage or the UV emissions? An explanation would have been helpful.
Good description
Good description
Excellent post, really
Excellent post, really helpful thanks
Add a comment