Atoms in pure metals arrange into regular patterns when they solidify from their liquid state, and these replicate various geometries. Depending on the arrangement taken up by the atoms, they can be classified under various structural types, better known as crystal structures.
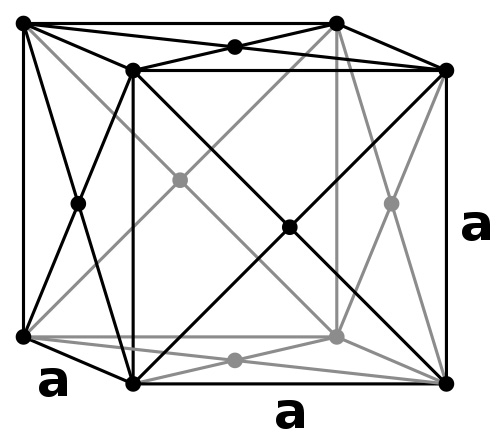 The most common crystal structures are either cubic or hexagonal. If other elements are added to pure metals, they can form a type of metallic alloy called a solid solution; this is much like a solution made by dissolving salt in water, but only in the solid form. In a solid solution, the foreign atoms can secure suitable positions or sites within the virgin metal and still maintain an ordered structure.
The most common crystal structures are either cubic or hexagonal. If other elements are added to pure metals, they can form a type of metallic alloy called a solid solution; this is much like a solution made by dissolving salt in water, but only in the solid form. In a solid solution, the foreign atoms can secure suitable positions or sites within the virgin metal and still maintain an ordered structure.
Another class of widely known metallic alloys is the intermetallics; in fact, these constitute the largest class of materials known today. An intermetallic, or intermetallic compound, forms when two or more elements are mixed in specific proportion; or in other words, they form with distinct chemical formulas. Just as in pure metals, the different atoms in intermetallic compounds form a regular periodic array. With regards to their properties, intermetallics fall somewhere between metals and ceramics. They are stronger and more resistant to oxidation (environmental attack, like rusting of iron) than metals, but generally not as much as ceramics. Although, most intermetallics show poor ductility and shatter like ceramics, they have some considerable advantages in terms of compatibility and processing.
 Interestingly, intermetallic compounds have been known to humans since as far back as back as 2500 B.C., when a copper-arsenic (Cu3As) compound was used in Egypt and Anatolia as a coating for strengthening metallic tools. Other instances of ancient usage of intemetallics can be cited to yellow brass (copper-zinc, CuZn) for coins and ornaments, and silver-tin amalgams (compounds of mercury) for dental fillings. Centuries of research and development has led to the discovery of various modern intermetallic compounds with properties strikingly different from the parent metals. Nickel-titanium (NiTi) intermetallics display the shape-memory effect, whereby after being deformed it reverts back to the original shape upon heating; these materials are used for noise-reduction features in jet engines and for stents in surgical applications. Nickel-iron (Ni3Fe) and cobalt-samarium (Co5Sm) are some of the strongest magnets available and are used in sophisticated musical instruments and microphones. Zirconium-aluminium (Zr3Al) intermetallics display high resistance to radiation damage and are used extensively in nuclear reactors.
Interestingly, intermetallic compounds have been known to humans since as far back as back as 2500 B.C., when a copper-arsenic (Cu3As) compound was used in Egypt and Anatolia as a coating for strengthening metallic tools. Other instances of ancient usage of intemetallics can be cited to yellow brass (copper-zinc, CuZn) for coins and ornaments, and silver-tin amalgams (compounds of mercury) for dental fillings. Centuries of research and development has led to the discovery of various modern intermetallic compounds with properties strikingly different from the parent metals. Nickel-titanium (NiTi) intermetallics display the shape-memory effect, whereby after being deformed it reverts back to the original shape upon heating; these materials are used for noise-reduction features in jet engines and for stents in surgical applications. Nickel-iron (Ni3Fe) and cobalt-samarium (Co5Sm) are some of the strongest magnets available and are used in sophisticated musical instruments and microphones. Zirconium-aluminium (Zr3Al) intermetallics display high resistance to radiation damage and are used extensively in nuclear reactors.
Another very important application for intermetallic compounds is their use in the gas and jet turbine engines. The rotating components in the rear sections of the engines can experience temperatures in excess of 1500 °C coupled with high loading stresses. Currently, the materials used comprehensively for this demanding application are nickel-based superalloys.
Strikingly, one of the major components of the superalloys is an intermetallic that is formed between nickel and aluminium, Ni3Al. It is this component, or phase, which is responsible for the high strengths that these alloys demonstrate at high temperatures. It was back in late 1950s when workers in General Electric Company, New York, unravelled the extraordinary property of this intermetallic phase. Unlike common metals and alloys that become weaker with increasing temperatures, the strength of this intermetallic actually increased with increasing temperatures. This anomalous behaviour was seen up to 700°C, where the strength was doubled compared to at room temperature. By incorporating further modifications in the composition and processing parameters, an advanced nickel-base superalloy today shows peak strength at about 800 °C. Strength then drops as temperatures go even higher, but the material still retains adequate strength to endure extreme conditions.
The remarkable behaviour of Ni3Al sparked tremendous enthusiasm amongst materials scientists which led to active worldwide research on Ni3Al and similar intermetallics for high temperature applications. Researchers, since then, have found compounds like nickel-silicon (Ni3Si), titanium-aluminide (TiAl), iron-aluminide (FeAl) and cobalt-titanium (Co3Ti) that behave similarly. However, retaining significant strength at high temperatures is not the only criterion that a structural intermetallic has to fulfil - it also has to be more damage tolerant or ductile at room temperature, and should also show good oxidation resistance. In the case of Ni3Al, the issue of poor ductility was circumvented by additions of tiny quantities of boron, as little 0.5 milligrams in 1 kilogram (i.e. 500 parts per million, by weight). With boron 'doping' Ni3Al became so ductile it could be stretched by a phenomenal 35%, which is comparable to some pure metals. With regards to oxidation, the aluminium from Ni3Al reacts with atmospheric oxygen at high temperatures to form a continuous oxide layer (called alumina, Al2O3) on the surface, which then protects the rest of the component from further environmental attack.
 Similar to Ni3Al, other intermetallics such as Ni3Si display increasing strength with temperature. However, Ni3Si and FeAl show poor low temperature ductility, which poses problems with fabrication. Co3Ti shows substantial promise, but it lacks in high temperature oxidation resistance as the oxide layer it forms is not protective. Some other candidates display good oxidation resistance, such as molybdenum-silicon (MoSi2), but poor high temperature strength. One particular intermetallic, molybdenum-silicon-boron (Mo5SiB2) has shown exceptional resistance against oxidation up to temperatures of 1700 °C, but its brittleness still remains an obstacle.
Similar to Ni3Al, other intermetallics such as Ni3Si display increasing strength with temperature. However, Ni3Si and FeAl show poor low temperature ductility, which poses problems with fabrication. Co3Ti shows substantial promise, but it lacks in high temperature oxidation resistance as the oxide layer it forms is not protective. Some other candidates display good oxidation resistance, such as molybdenum-silicon (MoSi2), but poor high temperature strength. One particular intermetallic, molybdenum-silicon-boron (Mo5SiB2) has shown exceptional resistance against oxidation up to temperatures of 1700 °C, but its brittleness still remains an obstacle.
TiAl, however, has shown the brightest prospect with an optimal balance of properties. Along with the protective oxide layer it has the added advantage of being lighter than Ni3Al, which is essential for the aerospace industry. In fact, TiAl is already used for some components of the new generation of turbine engines, however their temperature capability is still inferior to Ni3Al based superalloys.
At present, materials scientists all over the globe are in constant hunt for an advanced intermetallic compound that would be capable of superseding the properties of the current nickel based superalloys. The search has now been going on for almost two decades, and its pace has picked up over the last five years in order to cope with the demanding design criteria of newer generations of gas or jet turbine engines.
A new high temperature aerospace material has proved to be elusive so far, however, the candidate materials have to ascend from this peculiar and exciting class of materials, the intermetallics.









Comments
Add a comment