In the middle of the night we sleep soundly knowing that if something in our house goes awry we'll be warned. If smoke fills the downstairs and threatens the air we breathe, an 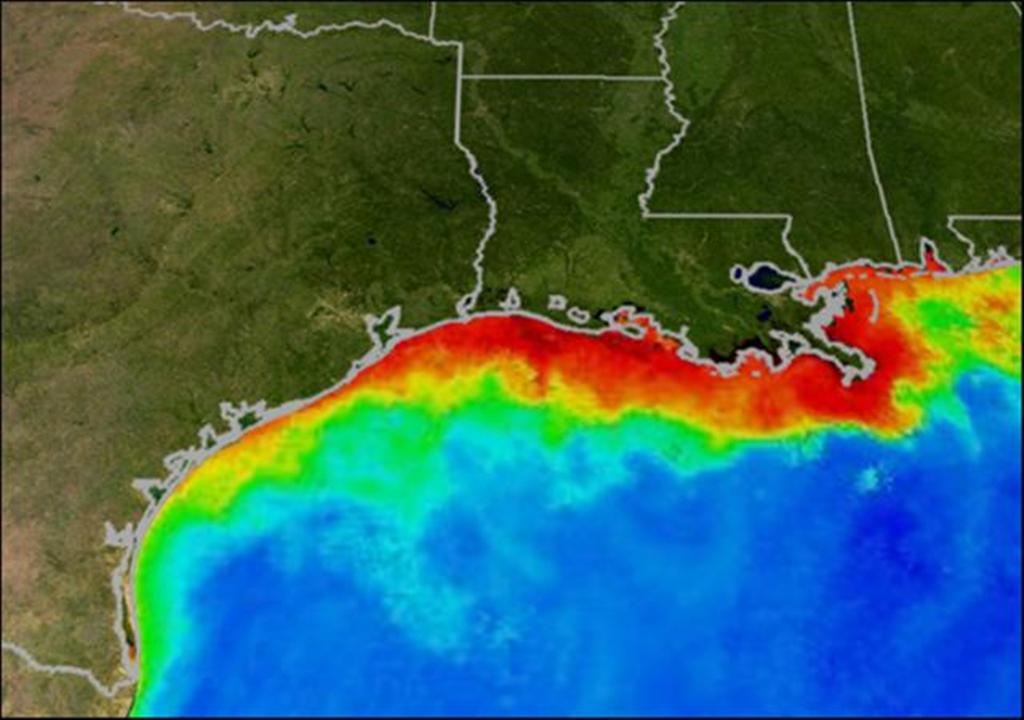 alarm will sound. There are alarms for odourless toxic gases, for windows broken by would-be thieves and even alarms to tell us when the washing's done. But imagine if there weren't. What if, when we lay our heads upon our pillows, we had no way of knowing what we might breathe? Does this sound dramatic? You bet it does. But if you lived in the ocean, and the sea floor were your home, this would be your reality.
alarm will sound. There are alarms for odourless toxic gases, for windows broken by would-be thieves and even alarms to tell us when the washing's done. But imagine if there weren't. What if, when we lay our heads upon our pillows, we had no way of knowing what we might breathe? Does this sound dramatic? You bet it does. But if you lived in the ocean, and the sea floor were your home, this would be your reality.
In my previous two essays (see below for links) we have learned that excess nitrogen can lead to increased phytoplankton growth. And when these small microscopic plants die and decay, oxygen in the water column is consumed causing hypoxia (low oxygen) and even anoxia (no oxygen). In some cases this is a natural occurrence, but in many cases it is not and the blame rests with us. Indeed, persistent low-oxygen conditions are developing in various places throughout our oceans and particularly near coasts where humans add nitrogen (mostly in the form of fertilisers washed off the land) to the sea.
If these low oxygen conditions persist they can trigger a phenomenon called habitat compression. This is where the area of hypoxia grows, restricting the remaining habitable space available for organisms to live in. If you are a mobile organism, like a fish, you may be able to escape and there are vivid accounts of animals moving into the shallows and even crawling to the tops of dock pilings to avoid underlying hypoxic conditions. But if you are a sessile animal and unable to move, like a mussel that must remain attached to something, then you are stuck and left to endure the low oxygen event. In severe cases these events cause massive die-offs of marine organisms, known appropriately as "fish kills", and they ultimately decrease ecosystem biodiversity. Dramatic events like these aren't the only way that the ecosystem may be impacted. Less severe, persistent or frequent low oxygen events can also greatly alter the environment. In this case, the organisms exposed to hypoxia grow and reproduce less and are more easily preyed upon.
As a general rule, organisms that inhabit the sediment or benthos have it worst of all. When you dwell on the bottom you live at the greatest distance from the atmosphere, which is a major source of aquatic oxygen replenishment. In addition, marine sediments are generally lower in oxygen compared with the water column above them. And because sediments are typically anoxic below the top few centimeters (or often millimeters), benthic organisms already live on the edge. So any hypoxic event can easily push them past the point of survival.
Of course, low oxygen concentrations don't just impact multi-celled, 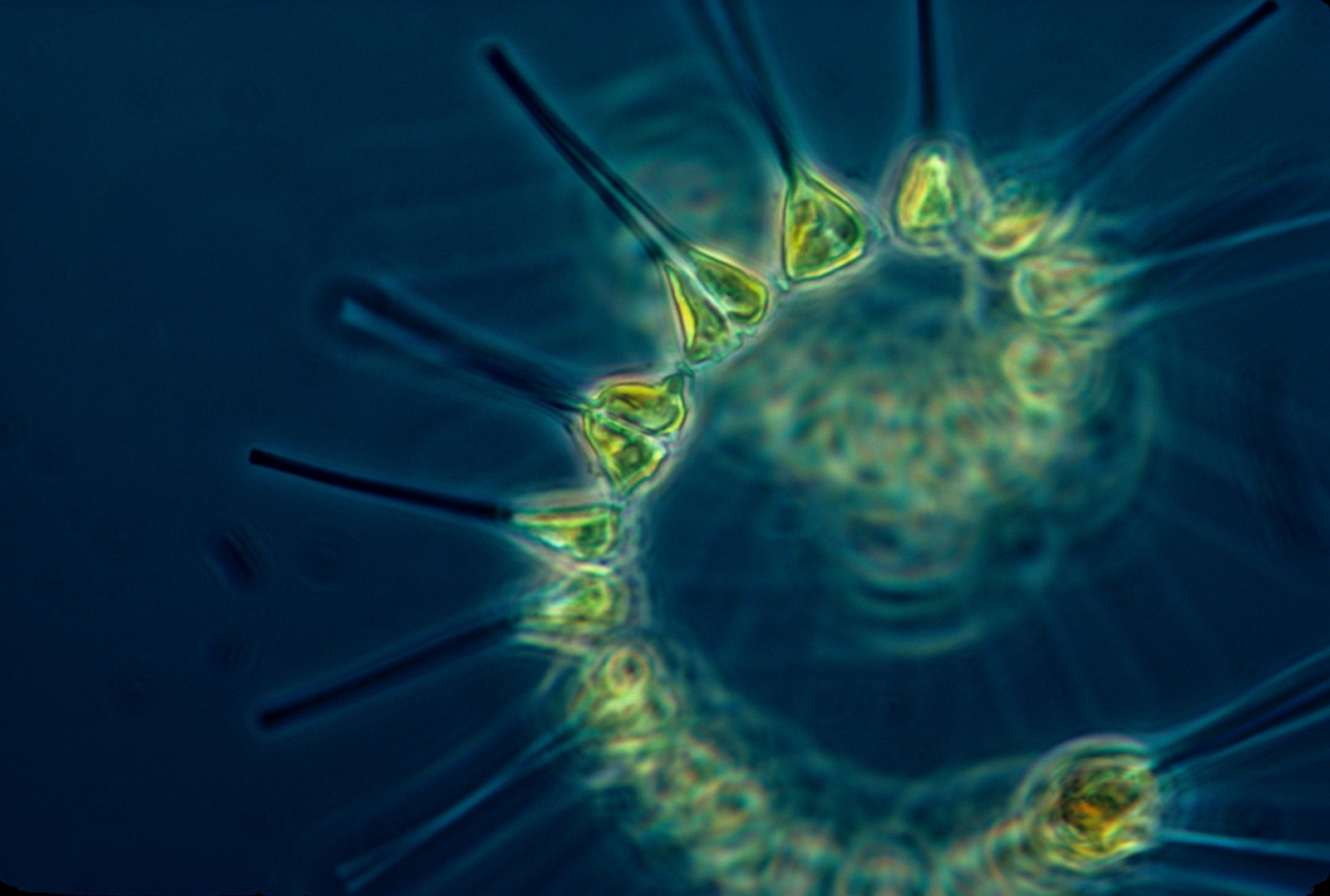 visible organisms - the microbial community can be hard hit too. Microbes are amazing organisms that can tolerate extreme conditions that range from hot springs and hydrothermal vents to glacial ice and polar bear fur. They can consume wood, plastics, oil and even radioactive materials, and one of their critical roles in the ocean is the removal of nitrogen from the water. This process converts a form of nitrogen called nitrate (NO3-), which is the form usually found in fertilisers and is readily consumed by phytoplankton, into di-nitrogen gas (N2), a form which is unuseable by most organisms. This natural nitrogen filter, known as denitrification, can remove a significant portion of the nitrogen we discharge into the coastal ocean. It also occurs under anaerobic (in other words no-oxygen) conditions.
visible organisms - the microbial community can be hard hit too. Microbes are amazing organisms that can tolerate extreme conditions that range from hot springs and hydrothermal vents to glacial ice and polar bear fur. They can consume wood, plastics, oil and even radioactive materials, and one of their critical roles in the ocean is the removal of nitrogen from the water. This process converts a form of nitrogen called nitrate (NO3-), which is the form usually found in fertilisers and is readily consumed by phytoplankton, into di-nitrogen gas (N2), a form which is unuseable by most organisms. This natural nitrogen filter, known as denitrification, can remove a significant portion of the nitrogen we discharge into the coastal ocean. It also occurs under anaerobic (in other words no-oxygen) conditions.
So now you are thinking, "this is perfect - we have a way to clean up the nitrogen pollution we add to the environment and which needs no oxygen to work; less nitrogen in the water will decrease excess phytoplankton growth and, subsequently, with less organic matter to decompose, more oxygen will remain in the water column, and dead zones should be a thing of the past."
If this was your thought process, you were partly correct. But there is one critical step missing. Denitrification needs nitrate (NO3-); once the nitrate is gone, the process shuts down. And unfortunately, in many systems, nitrate comes from an aerobic (or oxygen requiring) process called nitrification. In nitrification, ammonium ions (NH4+) are converted to nitrate (NO3-) ions, making nitrate available for denitrification. And if the nitrifying microbes stop making nitrate then denitrification halts. This leaves ammonium lurking in the system to fuel future phytoplankton blooms, exacerbating the problem.
That would be bad enough, but it gets more complicated! Both nitrification and denitrification can produce nitrous oxide (N2O). Nitrous oxide is more commonly known as laughing gas, but I assure you in this case it's no laughing matter. This colourless gas has a heat-trapping (greenhouse gas) potential over 300 times more powerful than carbon dioxide, so it can contribute to global warming. In addition, it reacts in the atmosphere to deplete the ozone layer. Human activities, such as fossil fuel burning and fertiliser application, lead directly to N2O emissions, but low oxygen conditions in the marine environment also lead to the production of the gas. Research has shown that frequent flip flops between oxygen replete and oxygen depleted conditions can cause increased N2O emissions from both nitrification and denitrification. And work is currently underway to see if consistent oxygen-devoid environments, like dead zones, do the same thing.
So, is all lost? Tonight, when we lay our heads upon our pillows, should we feel sad and hopeless? The answer is a resounding, "NO!" First, it is interesting to remember that a dead zone isn't really dead. It turns out that there are numerous microbes that survive and in some cases thrive under hypoxic and anoxic conditions. They might not be the ones that come readily to mind, but that life survives under various conditions is a comforting thought. Second, and most important, there are simple everyday actions we can do to decrease our nitrogen footprint. By decreasing the amount of nitrogen in the environment we lessen our impact on the ocean. Want to know what to do? Stay tuned for the next article, where I'll explain...










Comments
Add a comment