Working at temperatures close to the melting point of the material and spinning hundreds of times per second while simultaneously supporting a load equivalent to the weight of a family car, the blades in a modern jet engine have to withstand what is arguably one of the most extreme environments any engineered substance could ever encounter. The combustion products routinely reach temperatures of 2000°C, and the environment inside the engine is highly corrosive and oxidising. So how have designers found substances capable of beating these combined mechanical, chemical and thermal odds?
 | 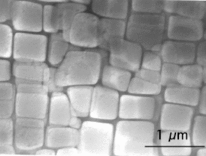 |
| Under the microscope, superalloys look like an aerial view of San Francisco. | Electron micrograph of a superalloy |
The answer lies in a family of materials known as superalloys, a name that is entirely justified because they are some of the most impressive substances ever synthesised. They are typically based on the metal nickel, an element that is relatively light, cost-effective to use and resistant to oxidation. It also has a melting temperature of 1455°C, which means that things made from it can operate at very high temperatures. But impressive as nickel is on its own, it's when it's combined as an alloy with other elements that it really comes into its own.
Alloying, which simply means adding small amounts of other elements, can dramatically alter the properties of a material, a trick that metallurgists have been aware of for centuries. Adding tin to copper ushered in the Bronze Age by about 2500BC, and later the Romans and ancient Chinese used mercury-tin amalgams to gild mirrors and armour; mercury-mixtures were also employed by medieval miners to extract precious metals from their ores.
 |
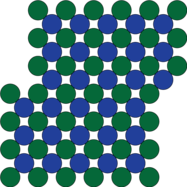
| The crystal structure of a superalloy; the material in the 'road' (see photo and microscope image). |
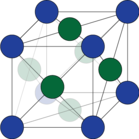 |
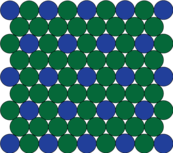
| The crystal structure in the 'buildings' of the superalloy structures (see photo/microscope image). |
 |
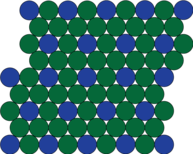
| Showing the cube face of a superalloy 'building' crystal. |
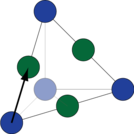 |
| Showing the diagonal face of the superalloy 'building' crystal. |
But what sets modern-day materials apart from those tried and tested by time is that, today, we understand much more, at an atomic level, about why alloys work. Some of the theories developed over the last 50 years, including techniques like quantum mechanics, are now beginning to provide useful insights into how alloying will affect the structures and properties of the resulting materials, and while these methods are being perfected, there is also over sixty years of empirical data to fall back on, including statistical methods that can deduce how a new material might behave. That said, designing new alloys certainly isn't easy, because predicting how atoms of different elements will behave and interact with each other when they are mixed is extremely challenging.
However, in the case of the nickel-based superalloys, a lot of progress has been made. A key breakthrough has come with the inclusion of aluminium. When this is added to nickel, metallurgists have found that the resulting material gets stronger as the temperature rises. But what do we actually mean by strength? Well, if a force is applied to a metal causing it to stretch, up to a certain point the metal will spring back to its original shape. But beyond a certain loading level, the metal begins to deform, permanently. We call this the yield stress of a metal. In the case of most materials, the yield stress falls as the temperature rises because, when a metal is heated, it becomes softer. But with aluminium-nickel superalloys, this yield stress initially remains constant, and then starts to increase, reaching a maximum at around 700°C.
Fortuitously, this is exactly the sort of temperature where extra strength is needed in a turbine engine. But why does this happen? It's because, rather like the dregs of a sweet cup of coffee which forms sugar crystals as the residual liquid cools, in a cooling alloy small particles begin to precipitate in the metal, forming small particles embedded throughout the material. Seen down the electron microscope, they resemble small cubes that arrange themselves a bit like the buildings and roads in a large American city seen from the air.
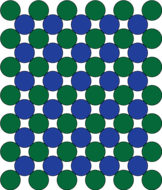 |  |
| The cube face of the crystal structure of a superalloy, before slip happens. | After the cube face has slipped, the blue and the green atoms end up in the identical environment. |
 |  |
| The diagonal face of a crystal, when extended out. | After slip on the diagonal face, the blue atoms get closer together. This is a defect, called an anti-phase boundary, and comes with an energy penalty. It is this defect that makes the superalloys so good at high temperatures. |
But, at the atomic level, what actually are these building and road-like structures and how do they affect the behaviour of the material? Surprising as it might sound, metals are actually made up of crystals, or even, in the case of most engineering metals, crystals within crystals. And in a superalloy, the "buildings" and the "roads" are metal atoms arranged into different configurations or crystal structures.
The "road" is made of a so-called "face-centered cubic" (FCC) structure. This is the most common structure found in metal crystals. Imagine a die with all the faces showing 5 spots: if there were an atom at each spot, this would look like the atoms in a face centered cubic structure, which we represent it by the greek letter "γ" and refer to as the gamma phase.
The "buildings" are a bit different. At the right concentration and temperature, the aluminium atoms spontaneously arrange themselves so that they sit at the corners of each of the faces of the die. Deceivingly, it can look like there are more aluminium atoms that nickel atoms but, in fact, there are actually three times as many nickel atoms. This is because when these dice are stacked up to form a crystal, each atom at the corner is shared with 8 other cubes, but the atoms in the centres of the faces are shared with only one other cube. Since this structure shares similarities with the gamma structure, we call this the gamma prime, or γ'.
But why is this important? The high temperature strength of a superalloy stems from the way these crystals behave. When materials permanently deform, atoms slide past each other. This happens on specific planes and directions in the crystal. To think about the planes, we can look at the face, or a plane cutting through the cube along the diagonals.
When we extend these out into a larger crystal, these planes look quite different: one is closely packed, and the other is more open. The closely packed diagonal planes can slip past one another very easily, but the more open planes can't.
Indeed, for most face centred cubic materials, the most closely packed layers of atoms can slip past each other. But in the γ' configuration this can't happen so easily. In these crystals, if one layer of closely packed atoms slips over the layer below, the blue aluminium atoms move closer toward each other, which is energetically unfavourable. In contrast, when the layers of atoms slip past each other on the more open, face planes, the aluminium atoms stay apart.
At higher temperatures, the energetically more favourable but "jerkier" motion on the faces of the cube becomes possible due to the increased thermal motion of the atoms. But the transition between these two regimes leads to debris in the material that stops atoms slipping past each other. As a result, more force is needed to push atoms around and the material becomes stronger at higher temperatures.
This effect is the main reason that superalloys have enjoyed such success as high temperature materials; but it also leads to a problem. Most components are formed by mechanically working a material into a shape at high temperature. But for a superalloy, this gets impossibly hard. As a result, the processing of superalloys requires advanced approaches: colossal forging presses or careful solidification is crucial in making useful components from these remarkable materials.
- Previous Making Metals Stronger
- Next The Ocean's Cleaners










Comments
Add a comment