Networking Brain Cells
Interview with
So, we can examine what's gone wrong in the brains of patients that have had 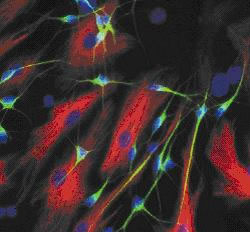 Huntington's and donated their brains after passing away. But there's also other ways that scientists can study the samples. Professor Mike Dragunow is able to culture stem cells that he gets from the brain bank. He also gets brain tissue donated from epilepsy patients who have elected to have surgery to cut out small parts of their brain to help control their seizures. He harvests this tissue and grows the adult human brain cells in a Petri dish to form a network of brain cells.
Huntington's and donated their brains after passing away. But there's also other ways that scientists can study the samples. Professor Mike Dragunow is able to culture stem cells that he gets from the brain bank. He also gets brain tissue donated from epilepsy patients who have elected to have surgery to cut out small parts of their brain to help control their seizures. He harvests this tissue and grows the adult human brain cells in a Petri dish to form a network of brain cells.
Hannah Critchlow asked him, how similar are these cells to a conscious, real living brain. Is this a better approach to studying the brain than by looking in sheep or rats? And can you really compare a culture system to how a fully functional living brain actually works?
Mike - Well, it's always a challenge actually because once you grow them in a dish, you already have a very much artificial environment. But what we can do is we can use markers for the di fferent cell types that we know are present in the brain and see whether those markers exist in the cells in the dishes, and they do. And so, what we're trying to do really is understand how those human brain cells function, their basic biology, because we can test that. So, we're looking at understanding how human brain cells that are involved in a number of neurological disorders, how they behave, their biochemistry. And also, we're testing compounds directly on those human brain cells. Now, one of the big problems in the neurosciences really is that compounds developed in model systems haven't actually translated to humans. So, you have medications that work wonderfully in transgenic models of Alzheimer's disease in mice for example that cure the mice of Alzheimer's and yet, so far at least, none of those compounds have worked effectively in humans with Alzheimer's.
And the approach we're taking I suppose is to try and take a more direct approach if we can actually understand first the biology of the cells and secondly, use those cells to test drugs. The drugs that work on the human brain cells in the dish may - we don't know if they will, but they may be effective in real humans that are living and walking around. So, what we've been doing really is learning how to grow the cells, defining the different types of cells and some cells grow better than others - the dividing cell population that we do freeze down because they bulk up, it turns out that these dividing cells - we're not 100% sure. We're about 95% sure now through a number of different genetic techniques in fax sorting...
Hannah - Sorry, fax sorting. Is that fax as in like faxing something, f transfer of information there?
Mike - No, it's a sort of flow cytometry. It allows you to actually sort cells on the basis of certain markers on their surface. We use that to try and identify what sort of cells you have. Using these approaches, we think that pretty sure these cells that we're growing in bulk here are called brain pericytes. Now the pericyte actually is a very old cell, but a very, very understudied, and under understood cell really because what it does, it actually lines blood vessels. In the brain actually, there's actually a large number of pericytes and one of their functions in the brain to line blood vessels is to maintain the blood-brain barrier. Now, this barrier is very important because molecules in the body and the blood, there are certain molecules you don't want to get into the brain. And so, the brain has this privilege, this blood-brain barrier, the pericytes maintain the barrier and we now know through work by a number of researchers around the world that the blood-brain barrier breaks down in a number of neurological disorders especially in Alzheimer's.
Hannah - So, these pericytes in this blood-brain barrier really stops any toxins entering the brain that might cause damage to the brain and therefore, cause any behavioural problems like seen in Alzheimer's or Huntington's for example.
Mike - That's right, yes and so, people think that once that barrier breaks down, toxins can get into the brain. They can actually add directly to the nerve cell degeneration but they can also activate immune cells in the brain to cause inflammation. We need to study more of the human versions of these inflammatory cells in the brain, the microglia and the astrocytes directly.
Hannah - Which is why it's so important to get these samples from the human brain bank tissue and also from the surgery.
Mike - It's amazing really and our work really is driven by the amazing generosity of the patients who are undergoing surgery and also, the donations that people with fatal brain disorders give to the brain bank. It's incredible and their families. They really drive our work and they also motivate you as a scientist. When you're studying the human brain or human brain cells, you're much more motivated to try and understand and provide some useful answers. And so, that's our approach really, by studying human brain cells by testing drugs directly on them. Our hope is to identify molecules that will work in humans to help reduce and stop the progression of some of these terrible disorders.
Hannah - So, a whole host of brain disorders show an altered immune system, where the brain is effectively being attacked by its body's own defence system. Cases of Schizophrenia, depression, Alzheimer's, and Huntington's all involve the brain being almost on fire with inflammation. And Mike is now working on trying to come up with molecules that will dampen down this heightened inflamed response and repair the brain in the hope that this will lead to new treatments for patients. Next, I speak with Professor Richard Faull who set up this brain research centre at Auckland University to find out how researching Huntington's disease is also helping us to grasp the incredible scale of complexity of the human brain. He tells us what decades of research into the disease have taught him.
Richard - So, Huntington's disease was generally thought to be a simple disease because it was caused by one gene and why there has been so much attention on this gene scientifically and on this disease, was the simple idea that if we could solve this disease by working out what this one gene defect caused, then we could solve diseases like Alzheimer's, Parkinson's, and Motor Neurone Disease which are multiple gene diseases. Well, it just so happens that we now know that this single-gene disease, Huntington's disease is a very complicated disease because it actually causes disregulation and upset of at least a quarter of all the genes. We have 45,000 genes in each cell and depending on what part of the brain it is, will upset different combinations of these other genes you see, that's why you get all the different symptoms. And so, genetics is a very complex science now. We thought it was a simple science when I was at school and that this gene would do just one thing. It doesn't. The gene makes a protein, that protein interacts with other genes, and causes them to change their function or change their pattern of protein production. So, there is a sort of an effect which spreads like wild fire in variable ways. And the variation is affected by the environment. And so, we know that people in different environments, even with the same gene will result in different patterns of brain degeneration and symptoms. And Huntington's disease has taught us the fundamental principle that the human brain is more complex than what we ever, ever imagined. We can't explain a human thought. We can't explain what a sudden burst of genius is, billion neurons talking to one another in complicated ways gives us the conscious personality and gives us and makes us what we are, and we're beginning to unravel that. But it's almost as if we climb to the top of Mt. Everest, thinking we're going to solve this disease and then we see all the Himalayas before us which are even higher. And that's the challenge of doing brain research. It's going to be several lifetimes of work to unravel the human brain. And this disease has actually led us along that path.
- Previous The gene for Huntington's
- Next Living with Huntington's disease










Comments
Add a comment