A New Element - Ununbium
Interview with
Chris - And also this week scientists have come up with a reason for you to tear up that periodic table which is on the wall of your chemistry laboratory or your school classroom, and replace it with a new one. This is because we have a new element to add to it. And here to tell us about that new element is someone who occasionally contributes to the Naked Scientists, but is also a BBC science reporter, and that's Victoria Gill.
So, why have we got this new element Victoria?
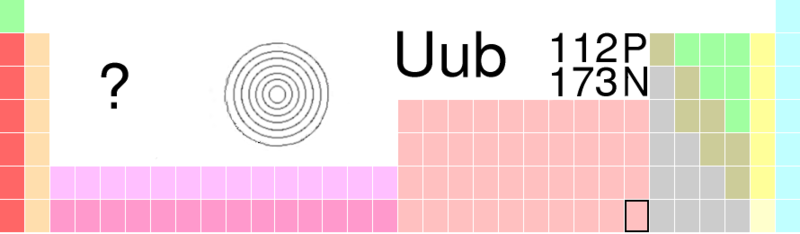 Victoria - Well this is element 112 or Ununbium, called that because its atomic mass, the mass of its nucleus is 112 [from the Latin un, un, bi - one, one, two]. It was discovered by Professor Sigurd Hofmann, in 1996 actually, but it was such a tricky experiment to replicate that it's taken all of this time for the International Union of Pure and Applied Chemistry [IUPAC], which is the official maker and formulator of our ubiquitous and wonderful periodic table to recognise it and credit Hofmann and his team at the Centre for Heavy Ion Research in Darmstadt, Germany, with it's discovery.
Victoria - Well this is element 112 or Ununbium, called that because its atomic mass, the mass of its nucleus is 112 [from the Latin un, un, bi - one, one, two]. It was discovered by Professor Sigurd Hofmann, in 1996 actually, but it was such a tricky experiment to replicate that it's taken all of this time for the International Union of Pure and Applied Chemistry [IUPAC], which is the official maker and formulator of our ubiquitous and wonderful periodic table to recognise it and credit Hofmann and his team at the Centre for Heavy Ion Research in Darmstadt, Germany, with it's discovery.
Chris - How did they actually make the new element, Victoria?
Victoria - So they're using a particle accelerator, and they're essentially firing a beam of ions at a target and fusing two nuclei together. This is a very tricky thing to do when you get to the very heavy elements of the periodic table because these fusion reactions require a lot of energy. To create element 112 or Ununbium as it's temporarily been known, they fired a beam of charged zinc atoms, or zinc ions, at lead atoms in the hope that some of them would fuse together and form a new element, and so they did. What's very tricky about this is that these elements are very unstable; as soon as they form they actually just start to fall apart. The nuclei start to emit energy, but that's quite useful because you can detect the energy that they're emitting and use that to estimate the size of the nucleus. So you can tell that you have a new element. But these fusion reactions don't happen very often, you have to fire this beam at these lead atoms for a very long time and you only get a few successful fusion reactions. In 1996 they only created or saw one atom of element 112. But other teams have had to replicate those experiments in order that IUPAC, the society that draws up our periodic table, can recognise that discovery and say "Yes, this is officially a new element, and we will add it to your periodic table."
Chris - So that's hardly a massive amount of money in the bank in terms of this, four atoms in the last twelve years. But where on the periodic table would we put this, if we were to add the square today, where would we be adding this?
Victoria - Well it's a metal - it would go underneath Mercury on the periodic table, that's where its square would be. In actual fact, because it's been around for so long, because we've known about it for so long other teams have done some experiments on it to find that its properties are very similar to that group and it fits quite nicely into that group.
Chris - Given that it hangs around for such a short space of time, I mean, looking at the half lives of some of the isotopes of element number 112, we're talking less than half a minute, why is this useful?
Victoria - Well this is about really finding out how atoms work and how matter works. And in actual fact what Professor Hofmann's team are doing in the longer term is looking for what they've referred to as "the island of stability". So they think there's a whole new class of elements which have electron shells much further out that are full, that will be able to hang around for much longer, so you're dealing with whole new groups of elements and matter that behaves in a completely different way.
Chris - And given, as you say, that they think there might be the prospects of getting very big elements, built the same way but way beyond the size of this one, could this therefore be used as something like a stepping stone, so you could build some of this and then very quickly add some more to it to get you into the realms of these very big atoms that might have all these exciting properties?
Victoria - That's right, because if, as we're seeing, atoms behave and are built in the way that we would expect, and these fusion reactions are working in the way that we expect, then we can incrementally build these experiments to carry out new fusion reactions and build atoms in exactly the same way, we just need bigger particle accelerators, better equipment and we can get there in the end, it's all just stepping stones as we say.
Chris - Thank you very much Victoria. That's Victoria Gill, explaining how the International Union of Pure and Applied Chemistry, also known as IUPAC for short, have confirmed the existence of a new element this week - was actually developed in the 1990's of course, but had to be proved to exist. They've given it the exciting name of Ununbium temporarily; that's un, un and bi in Latin. But, I'm told the IUPAC, they're going to be considering a new name for it, its official name in the next few weeks. They will listen to what the general public think too. So, if you got a name, you think that this element should have a certain name, tell us what you think and also, tell IUPAC as well.










Comments
Add a comment